We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox 123456
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
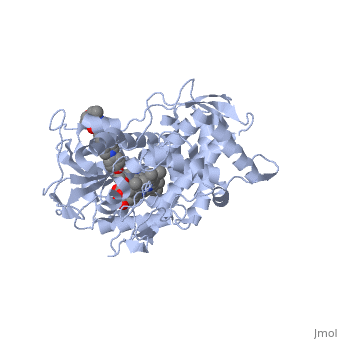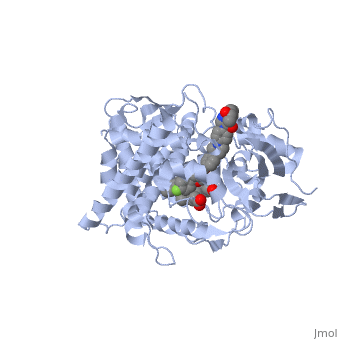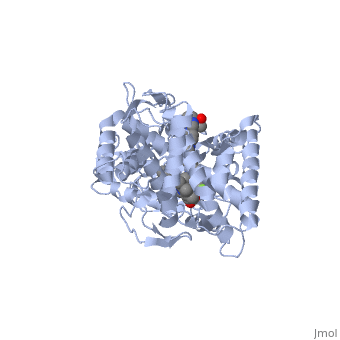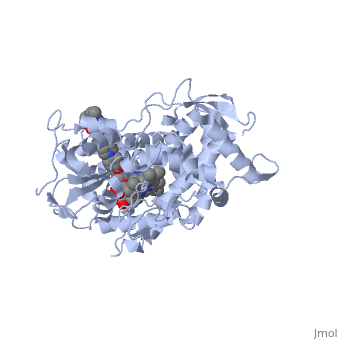
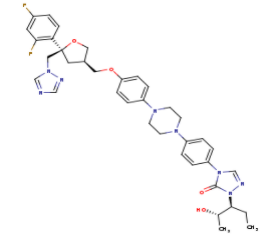
The primary component of Noxafil, posaconazole (green link) is a potent, broad-spectrum antifungal drug. It was derived from a similar triazole antifungal agent, Itraconazole. The differences in structure are that the chlorine substituents in the aromatic ring on the left-hand side of the images are replaces with fluorines and that the triazolone sidechain is hydroxylated in the posaconazole structure<ref name="drugbank">Posaconazole. (n.d.). Retrieved from https://www.drugbank.ca/drugs/DB01263 | The primary component of Noxafil, posaconazole (green link) is a potent, broad-spectrum antifungal drug. It was derived from a similar triazole antifungal agent, Itraconazole. The differences in structure are that the chlorine substituents in the aromatic ring on the left-hand side of the images are replaces with fluorines and that the triazolone sidechain is hydroxylated in the posaconazole structure<ref name="drugbank">Posaconazole. (n.d.). Retrieved from https://www.drugbank.ca/drugs/DB01263 | ||
| - | Accession Number: DB01263 </ref>. The extended side chain residues and hydrophobic contacts enhances antifungal activity by allowing tighter binding affinities to the <scene name='75/756730/Hemegroup/1'>heme cofactor</scene> in the active site of the CYP450-dependent enzyme lanosterol alpha-demthylase (CYP51) <ref name="groll">doi:10.1586/14787210.3.4.467</ref><ref>doi: 10.1086/523576</ref>. The tighter binding affinity of posaconazole makes it less susceptible to be affected by mutations in the enzyme resulting in resistance of fungi <ref name="formularyjournal">Sircar-Ramsewak,, F., Nicolau, D. P., & Kuti, J. L. (2005). Focus on posaconazole: A novel triazole antifungal for the treatment of invasive fungal infections. Formulary Journal - Modern Medicine Network </ref>. The scene shows the crystal <scene name='75/756730/ | + | Accession Number: DB01263 </ref>. The extended side chain residues and hydrophobic contacts enhances antifungal activity by allowing tighter binding affinities to the <scene name='75/756730/Hemegroup/1'>heme cofactor</scene> in the active site of the CYP450-dependent enzyme lanosterol alpha-demthylase (CYP51) <ref name="groll">doi:10.1586/14787210.3.4.467</ref><ref>doi: 10.1086/523576</ref>. The tighter binding affinity of posaconazole makes it less susceptible to be affected by mutations in the enzyme resulting in resistance of fungi <ref name="formularyjournal">Sircar-Ramsewak,, F., Nicolau, D. P., & Kuti, J. L. (2005). Focus on posaconazole: A novel triazole antifungal for the treatment of invasive fungal infections. Formulary Journal - Modern Medicine Network </ref>. The scene shows the crystal <scene name='75/756730/Structure/1'>structure</scene> of sterol 14-alpha demethylase (CYP51) from a pathogenic yeast Candida albicans in complex with the antifungal drug posaconazole |
Revision as of 20:52, 19 April 2017
| |||||||||||