We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox1996
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
== Structure == | == Structure == | ||
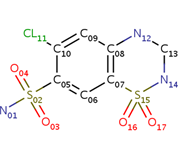
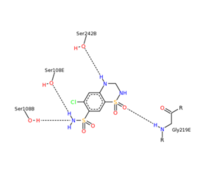
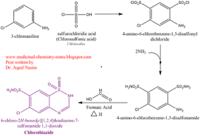
| - | <scene name='75/756546/Chlorothiazide/1'>Chlorothiazide</scene> is a semisynthetic chemical compound known chemically as 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide (Figure 1). It has a chemical formula of C<sub>7</sub>H<sub>8</sub>ClN<sub>3</sub>O<sub>4</sub>S<sub>2</sub> and a molecular weight of 298 Da <ref name = "one" > EMBL-EBI. (2012) 6-chloro-3,4-dihydro-2H-1, 2, 4-benzothiadiazine-7-sulfonamide-1, 1-dioxide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6/bound/HCZ </ref>. This chemical compound consists of a benzothiadiazine, sulfonamide, chloride, dihydro, and dioxide group. It has a melting point of 272 degrees Celsius, a flash point of 302.7 degrees Celsius, a solubility of 60 mg/ml in DMSO and less than 1 mg/ml in water, and appears as a white crystalline powder <ref name = "two" > Royal Society of Chemistry. (2015) Hydrochlorothiazide, ChemSpider. Retrieved from http://www.chemspider.com/Chemical-Structure.3513.html </ref>. The structure was determined by X-Ray diffraction and was measured at a resolution of 2.1 Angstroms <ref name = "three" > EMBL-EBI. (2012) Crystal structure of the AMPA subunit GluR2 bound to the allosteric modulator, chlorothiazide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6 </ref>. <scene name='75/756546/Drug/1'>Chlorothiazide was determined when bound to glutamate receptor 2</scene>. The enzyme cave of glutamate receptor 2 contained <scene name='75/756546/Inprogess/1'>specific amino acids</scene> that enabled binding of chlorothiazide. Binding involved hydrogen bonding between the <scene name='75/756546/Inprogess1/3'>nitrogen 12 and serine 242B, nitrogen 1 and serines 108B and 108E, and oxygen 17 and glycine 219 E</scene> (Figure 2). Synthesis of chlorothiazide occurs through the reaction between 3-chloroaniline, chlorosulfonic acid, and ammonia; and it is catalyzed by formic acid (Figure 3)<ref name = "four"> Nasim, A. (2017) Synthesis of chlorothiazide, Medical Chemistry Lecture Notes. Retrieved from http://medicinal-chemistry-notes.blogspot.com/2015/12/synthesis-of-chlorothiazide.html# | + | <scene name='75/756546/Chlorothiazide/1'>Chlorothiazide</scene> is a semisynthetic chemical compound known chemically as 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide (Figure 1) <ref name = "one" > EMBL-EBI. (2012) 6-chloro-3,4-dihydro-2H-1, 2, 4-benzothiadiazine-7-sulfonamide-1, 1-dioxide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6/bound/HCZ </ref>. It has a chemical formula of C<sub>7</sub>H<sub>8</sub>ClN<sub>3</sub>O<sub>4</sub>S<sub>2</sub> and a molecular weight of 298 Da <ref name = "one" > EMBL-EBI. (2012) 6-chloro-3,4-dihydro-2H-1, 2, 4-benzothiadiazine-7-sulfonamide-1, 1-dioxide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6/bound/HCZ </ref>. This chemical compound consists of a benzothiadiazine, sulfonamide, chloride, dihydro, and dioxide group. It has a melting point of 272 degrees Celsius, a flash point of 302.7 degrees Celsius, a solubility of 60 mg/ml in DMSO and less than 1 mg/ml in water, and appears as a white crystalline powder <ref name = "two" > Royal Society of Chemistry. (2015) Hydrochlorothiazide, ChemSpider. Retrieved from http://www.chemspider.com/Chemical-Structure.3513.html </ref>. The structure was determined by X-Ray diffraction and was measured at a resolution of 2.1 Angstroms <ref name = "three" > EMBL-EBI. (2012) Crystal structure of the AMPA subunit GluR2 bound to the allosteric modulator, chlorothiazide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6 </ref>. <scene name='75/756546/Drug/1'>Chlorothiazide was determined when bound to glutamate receptor 2</scene>. The enzyme cave of glutamate receptor 2 contained <scene name='75/756546/Inprogess/1'>specific amino acids</scene> that enabled binding of chlorothiazide. Binding involved hydrogen bonding between the <scene name='75/756546/Inprogess1/3'>nitrogen 12 and serine 242B, nitrogen 1 and serines 108B and 108E, and oxygen 17 and glycine 219 E</scene> (Figure 2). Synthesis of chlorothiazide occurs through the reaction between 3-chloroaniline, chlorosulfonic acid, and ammonia; and it is catalyzed by formic acid (Figure 3)<ref name = "four"> Nasim, A. (2017) Synthesis of chlorothiazide, Medical Chemistry Lecture Notes. Retrieved from http://medicinal-chemistry-notes.blogspot.com/2015/12/synthesis-of-chlorothiazide.html# |
</ref>. | </ref>. | ||
Revision as of 22:30, 19 April 2017
Diuril (Chlorothiazide)
| |||||||||||
References
- ↑ 1.0 1.1 EMBL-EBI. (2012) 6-chloro-3,4-dihydro-2H-1, 2, 4-benzothiadiazine-7-sulfonamide-1, 1-dioxide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6/bound/HCZ
- ↑ Royal Society of Chemistry. (2015) Hydrochlorothiazide, ChemSpider. Retrieved from http://www.chemspider.com/Chemical-Structure.3513.html
- ↑ EMBL-EBI. (2012) Crystal structure of the AMPA subunit GluR2 bound to the allosteric modulator, chlorothiazide, Protein Data Bank in Europe. Retrieved from http://www.ebi.ac.uk/pdbe/entry/pdb/3ik6
- ↑ Nasim, A. (2017) Synthesis of chlorothiazide, Medical Chemistry Lecture Notes. Retrieved from http://medicinal-chemistry-notes.blogspot.com/2015/12/synthesis-of-chlorothiazide.html#
- ↑ The Metabolomics Innovation Centre. (2016) Chlorothiazide, DrugBank. Retrieved from https://www.drugbank.ca/drugs/DB00880
- ↑ The Metabolomics Innovation Centre. (2016) Chlorothiazide, DrugBank. Retrieved from https://www.drugbank.ca/drugs/DB00880
- ↑ Freis, E., Wanko, A., Wilson, I., and Parrish, A.E. (1985) Treatment of essential diabetes with Chlorothiazide (Diuril), J. Am. Med. Assoc 166, 137-140
- ↑ Greene, J.A. (2005) Releasing the flood waters: diuril and the reshaping of hypertension, Bull. Hist. Med. 79, 749-794.
- ↑ 9.0 9.1 Drug.com. (2017) Diuril, Drugs.com. Retrieved from https://www.drugs.com/pro/diuril.html
- ↑ RxList Inc. (2017) Medical definition of diuretic, RxList: The Internet Drug Index. Retrieved from http://www.rxlist.com/script/main/art.asp?articlekey=7103
- ↑ Crawford, J.D., Kennedy, G.C., and Hill, L.E. (1960) Clinical results of treatment of diabetes insipidus with drugs of the chlorothiazide series, N. Engl. J. Med. 262, 737-743.
- ↑ 12.0 12.1 Simon, H. and Zieve, D. (2012) Kidney stones, University of Maryland Medical Center. Retrieved from http://umm.edu/health/medical/reports/articles/kidney-stones
- ↑ AHFS Patient Medication Information. (2017) Chlorothiazide, U.S. National Library of Medicine. Retrieved from https://medlineplus.gov/druginfo/meds/a682341.html
- ↑ The Mayo Clinic Staff. (2017) Idiopathic thrombocytopenic purpura (ITP), Mayo Clinic. Retrieved from http://www.mayoclinic.org/diseases-conditions/idiopathic-thrombocytopenic-purpura/symptoms-causes/dxc-20201224
- ↑ Jaffe, M.O. and Kierland, R. R. (1958) purpura due to chlorothiazide (Diuril), J. Am. Med. Assoc. 168, 2264-2265.