Function
Vorinostat (Zolinza) is an oral drug used as a cancer therapy to treat cutaneous T-cell lymphoma (CTCL) [1]. Vorinostat, previously known as suberoylanilide hydroxamic acid (SAHA), was the first HDAC inhibitor to be approved by the U.S. Food and Drug Administration on October 6, 2006 [2]. Cancer cells tend to over-express histone deacetylase (HDAC). HDAC removes acetyl groups on histones which results in a structural change of chromatin. This change in chromatin structure leads to the repression of gene expression which would result in the proliferation of cancer cells. is an HDAC inhibitor that ultimately stops the uncontrolled growth of the cancer cells. Vorinostat does this by inhibiting the function of HDAC by binding to its zinc-binding site and blocking the removal of acetyl groups. Vorinostat is taken once a day by mouth. The most common side effects were fatigue and gastrointestinal symptoms. [1]
Structure
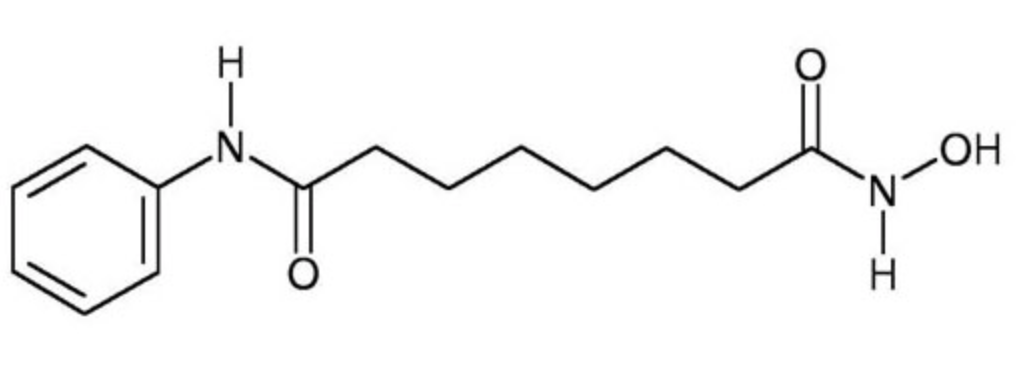
The IUPAC name for Zolinza is N'-hydroxy-N-phenyloctanediamide[3]. Vorinostat has a molecular formula of C14H20N2O3 and a molecular weight of 263.32 g/mol[4]. It has a melting point ranging from 159 to 160.5 degrees Celsius. This HDAC inhibitor is completely soluble in dimethylsulfide, somewhat soluble in water, ethanol, isopropanol, and acetone, and insoluble in methylene chloride[5]. It’s pH value in water solution is 6.6 with a pKa value of 9.2 [4]. All HDAC inhibitors consist of three different domains: a cap group that interacts with the binding pocket, a zinc binding domain (ZBD) that matches to the active site of the zinc ion, and a linker which binds the two together[6].
Mechanism
Zolinza is a potent nanomolar inhibitor of histone deacetylase (HDAC) activity, and this inhibition of activity can alternate the transcription of numerous genes via acetylation and transcription factors. It induces growth arrest, differentiation, or apoptosis in a variety of transformed cells. Zolinza inhibits HDAC activity by binding to the pocket of the catalytic site of the HDAC enzyme, and its hydroxamic acid moiety, an important moiety in the field of cancer therapy and a mutagenic agent, binds to a zinc atom of the enzyme with the rest of the molecule lying along the surface of the HDLP (histone deacetylase-like protein). Zolinza induces up to a nine-fold increase in p21WAF1 (a cell kinase inhibitor) mRNA and protein in T24 bladder carcinoma cells. This is caused by an increase in the rate of gene transcription associated with acetylation of the histones H3 and H4, associated with the p21WAF1 promoter. Zolinza also promotes the acetylation of numerous transcription factors like the androgen receptor, E2F-1, YY1, Smad7, EKLF, etc. In addition to histones and transcription factors, it also shows the acetylation of lysine residues of proteins, such as a-tubulin and protein Hsp90. Inhibition of HDAC6 activity leads to acetylation and the disruption of Hsp90, which leads to the decreased of progrowth activity and survival of proteins such as Bcr-Abl, mutant FLT-3, c-Raf and AKT in human leukaemia cells. Zolinza has the ability to influence the ability of tumor cells to undergo mitosis by disrupting the cell cycle and induce apoptosis of tumor cells by targeting cell cycle checkpoints. In vitro studies show that HDAC inhibitors create spindles that interfere with chromosome attachment, resulting in mitotic accumulation without affecting microtubules[4].
Disease
Lymphoma is a blood cancer that consists of lymphocytes (specific kinds of white blood cells) growing and multiplying without restriction[7]. These cancerous cells are then dispatched to the lymph nodes, blood, bone marrow and spleen, among other organs. The accumulation of these cells forms a tumor. One type of lymphocyte that can develop into a cancerous cell is T-lymphocytes (T-cells). An extremely prevalent type of T-cell lymphoma is cutaneous T-cell lymphoma (CTCL) which involves T-cell lymphomas that inflict damage on the skin. CTCL is a non-Hodgkin type of lymphoma that can also have negative impacts on the lymph nodes, blood, spleen, and other organs[8]. Patients often have dry skin accompanied with a rash and itching, as well as swollen lymph nodes. Patches, plaques, or tumors on the skin are characteristic of mycosis fungoides which is a mild type of CTCL. The majority of the time these symptoms are the only ones present, however, ten percent of patients that have later stages of the disease can develop severe complications. Sezary syndrome is a progressed form of mycosis fungoides that is identified by lymphoma cells appearing in the blood. This syndrome is systemic and chronic and has much more invasive symptoms such as red, itchy rashes covering over eighty percent of a patient’s body. Patients with this syndrome may also have patches and tumors on the skin, as well as alterations in hair, eyelids, nails, and enlargement of their lymph nodes. Zolinza is used to treat cutaneous manifestations that arise with CTCL, and this treatment is only recommended for patients that have undergone at least two systematic therapies with no improvement in their condition[5].
References
- ↑ 1.0 1.1 Duvic, M., & Vu, J. (2007). Update on the treatment of cutaneous T-cell lymphoma (CTCL): Focus on vorinostat. Biologics : Targets & Therapy, 1(4), 377–392. doi:https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2721288/
- ↑ National Cancer Institute. (2013). FDA Approval for Vorinostat. doi:https://www.cancer.gov/about-cancer/treatment/drugs/fda-vorinostat
- ↑ Grant, S., Easley, C., & Kirkpatrick, P. (2007) Fresh from the Pipline, Vorinostat. Nature Publishing Group. doi:http://www.nature.com/nrd/journal/v6/n1/full/nrd2227.html
- ↑ 4.0 4.1 4.2 Bubna, A. (2015) Vorinostat - An overview. Indian J Dermatol. 60:419.
doi:http://www.e-ijd.org/article.asp?issn=0019-5154;year=2015;volume=60;issue=4;spage=419;epage=419;aulast=Bubna
- ↑ 5.0 5.1 Merck & Co Inc. (2015). Highlights of Prescribing Information: Zolinza. doi:https://www.merck.com/product/usa/pi_circulars/z/zolinza/zolinza_pi.pdf
- ↑ Mottamal, M., Zheng, S., Huang, T. L., & Wang, G. (2015). Histone Deacetylase Inhibitors in Clinical Studies as Templates for New Anticancer Agents. Molecules, Vol 20, Iss 3, Pp 3898-3941. doi:www.mdpi.com/1420-3049/20/3/3898/pdf
- ↑ Lymphoma Research Foundation. (2012). Cutaneous T-Cell Lymphoma (CTCL). doi:http://www.lymphoma.org/site/pp.asp?c=bkLTKaOQLmK8E&b=6300151
- ↑ Pinter-Brown, L. C. & Schwartz, R. A. (2016). Cutaneous T-Cell Lymphoma. Medscape. doi:http://emedicine.medscape.com/article/2139720-overview