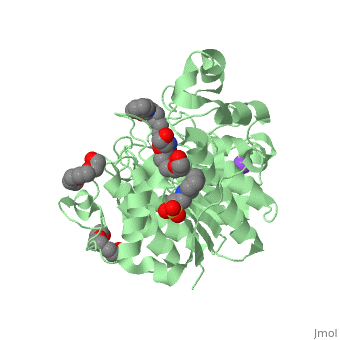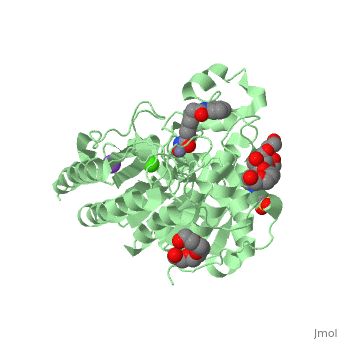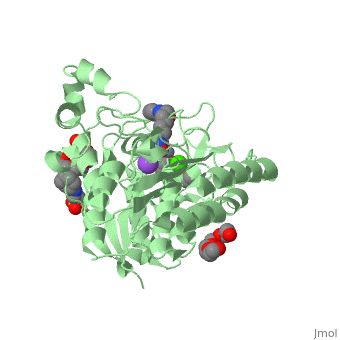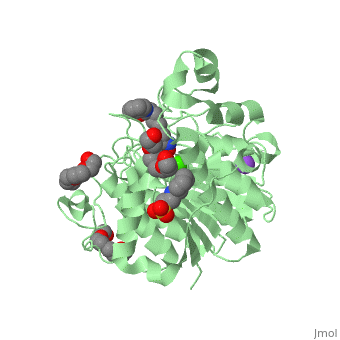Elizabeth A. Dunlap/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
The IUPAC name for Zolinza is N'-hydroxy-N-phenyloctanediamide<ref name="three">Grant, S., Easley, C., & Kirkpatrick, P. (2007) Fresh from the Pipline, Vorinostat. Nature Publishing Group. doi:http://www.nature.com/nrd/journal/v6/n1/full/nrd2227.html</ref>. Vorinostat has a molecular formula of C<sub>14</sub>H<sub>20</sub>N<sub>2</sub>O<sub>3</sub> and a molecular weight of 263.32 g/mol<ref name="four">Bubna, A. (2015) Vorinostat - An overview. Indian J Dermatol. 60:419. | The IUPAC name for Zolinza is N'-hydroxy-N-phenyloctanediamide<ref name="three">Grant, S., Easley, C., & Kirkpatrick, P. (2007) Fresh from the Pipline, Vorinostat. Nature Publishing Group. doi:http://www.nature.com/nrd/journal/v6/n1/full/nrd2227.html</ref>. Vorinostat has a molecular formula of C<sub>14</sub>H<sub>20</sub>N<sub>2</sub>O<sub>3</sub> and a molecular weight of 263.32 g/mol<ref name="four">Bubna, A. (2015) Vorinostat - An overview. Indian J Dermatol. 60:419. | ||
| - | doi:http://www.e-ijd.org/article.asp?issn=0019-5154;year=2015;volume=60;issue=4;spage=419;epage=419;aulast=Bubna</ref>. It has a melting point ranging from 159 to 160.5 degrees Celsius. This HDAC inhibitor is completely soluble in dimethylsulfide, somewhat soluble in water, ethanol, isopropanol, and acetone, and insoluble in methylene chloride<ref name="five">Merck & Co Inc. (2015). Highlights of Prescribing Information: Zolinza. doi:https://www.merck.com/product/usa/pi_circulars/z/zolinza/zolinza_pi.pdf</ref>. It’s pH value in water solution is 6.6 with a pKa value of 9.2 <ref name="four"/>. All HDAC inhibitors consist of three different domains: a cap group that interacts with the binding pocket, a zinc binding domain (ZBD) that matches to the active site of the zinc ion, and a linker which binds the two together<ref name="six">Mottamal, M., Zheng, S., Huang, T. L., & Wang, G. (2015). Histone Deacetylase Inhibitors in Clinical Studies as Templates for New Anticancer Agents. Molecules, Vol 20, Iss 3, Pp 3898-3941. doi:www.mdpi.com/1420-3049/20/3/3898/pdf</ref>. | + | doi:http://www.e-ijd.org/article.asp?issn=0019-5154;year=2015;volume=60;issue=4;spage=419;epage=419;aulast=Bubna</ref>. It has a melting point ranging from 159 to 160.5 degrees Celsius. This HDAC inhibitor is completely soluble in dimethylsulfide, somewhat soluble in water, ethanol, isopropanol, and acetone, and insoluble in methylene chloride<ref name="five">Merck & Co Inc. (2015). Highlights of Prescribing Information: Zolinza. doi:https://www.merck.com/product/usa/pi_circulars/z/zolinza/zolinza_pi.pdf</ref>. It’s pH value in water solution is 6.6 with a pKa value of 9.2 <ref name="four"/>. All HDAC inhibitors consist of three different domains: a cap group that interacts with the binding pocket, a zinc binding domain (ZBD) that matches to the active site of the zinc ion, and a linker which binds the two together<ref name="six">Mottamal, M., Zheng, S., Huang, T. L., & Wang, G. (2015). Histone Deacetylase Inhibitors in Clinical Studies as Templates for New Anticancer Agents. Molecules, Vol 20, Iss 3, Pp 3898-3941. doi:www.mdpi.com/1420-3049/20/3/3898/pdf</ref>. The <scene name='75/756751/Zn_and_vorinostat/1'>zinc ion</scene> that vorinostat binds to is shown in red while vorinostat is depicted as purple. |
== Mechanism == | == Mechanism == | ||
Revision as of 02:06, 20 April 2017
Zolinza
| |||||||||||