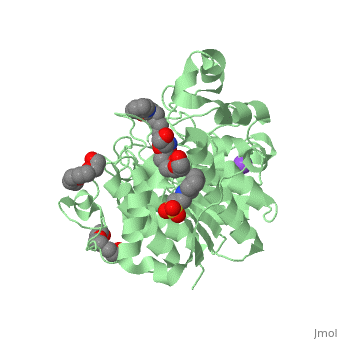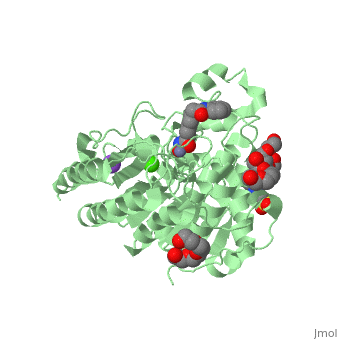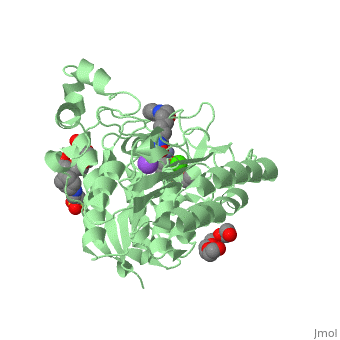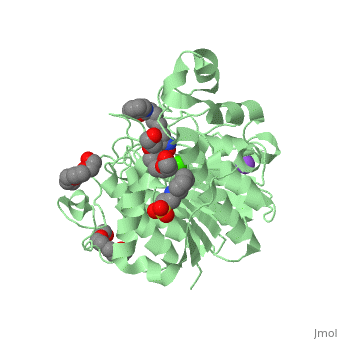Elizabeth A. Dunlap/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
== Mechanism == | == Mechanism == | ||
| - | Zolinza is a potent nanomolar inhibitor of histone deacetylase (HDAC) activity, and this inhibition of activity can alternate the transcription of numerous genes via acetylation and transcription factors. It induces growth arrest, differentiation, or apoptosis in a variety of transformed cells. Zolinza inhibits HDAC activity by binding to the pocket of the catalytic site of the HDAC enzyme, and its hydroxamic acid moiety, an important moiety in the field of cancer therapy and a mutagenic agent, binds to a zinc atom of the enzyme with the rest of the molecule lying along the surface of the HDLP (histone deacetylase-like protein). The <scene name='75/756751/Zn_and_vorinostat/1'>zinc atom</scene> that | + | Zolinza is a potent nanomolar inhibitor of histone deacetylase (HDAC) activity, and this inhibition of activity can alternate the transcription of numerous genes via acetylation and transcription factors. It induces growth arrest, differentiation, or apoptosis in a variety of transformed cells. Zolinza inhibits HDAC activity by binding to the pocket of the catalytic site of the HDAC enzyme, and its hydroxamic acid moiety, an important moiety in the field of cancer therapy and a mutagenic agent, binds to a zinc atom of the enzyme with the rest of the molecule lying along the surface of the HDLP (histone deacetylase-like protein). The <scene name='75/756751/Zn_and_vorinostat/1'>zinc atom</scene> that Zolinza binds to is shown in red while Zolinza is depicted as purple. |
Zolinza induces up to a nine-fold increase in p21WAF1 (a cell kinase inhibitor) mRNA and protein in T24 bladder carcinoma cells. This is caused by an increase in the rate of gene transcription associated with acetylation of the histones H3 and H4, associated with the p21WAF1 promoter. Zolinza also promotes the acetylation of numerous transcription factors like the androgen receptor, E2F-1, YY1, Smad7, EKLF, etc. In addition to histones and transcription factors, it also shows the acetylation of lysine residues of proteins, such as a-tubulin and protein Hsp90. Inhibition of HDAC6 activity leads to acetylation and the disruption of Hsp90, which leads to the decreased of progrowth activity and survival of proteins such as Bcr-Abl, mutant FLT-3, c-Raf and AKT in human leukaemia cells. Zolinza has the ability to influence the ability of tumor cells to undergo mitosis by disrupting the cell cycle and induce apoptosis of tumor cells by targeting cell cycle checkpoints. In vitro studies show that HDAC inhibitors create spindles that interfere with chromosome attachment, resulting in mitotic accumulation without affecting microtubules<ref name="four"/>. | Zolinza induces up to a nine-fold increase in p21WAF1 (a cell kinase inhibitor) mRNA and protein in T24 bladder carcinoma cells. This is caused by an increase in the rate of gene transcription associated with acetylation of the histones H3 and H4, associated with the p21WAF1 promoter. Zolinza also promotes the acetylation of numerous transcription factors like the androgen receptor, E2F-1, YY1, Smad7, EKLF, etc. In addition to histones and transcription factors, it also shows the acetylation of lysine residues of proteins, such as a-tubulin and protein Hsp90. Inhibition of HDAC6 activity leads to acetylation and the disruption of Hsp90, which leads to the decreased of progrowth activity and survival of proteins such as Bcr-Abl, mutant FLT-3, c-Raf and AKT in human leukaemia cells. Zolinza has the ability to influence the ability of tumor cells to undergo mitosis by disrupting the cell cycle and induce apoptosis of tumor cells by targeting cell cycle checkpoints. In vitro studies show that HDAC inhibitors create spindles that interfere with chromosome attachment, resulting in mitotic accumulation without affecting microtubules<ref name="four"/>. | ||
Revision as of 02:16, 20 April 2017
Zolinza
| |||||||||||