We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1240
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
== <b>Function</b> == | == <b>Function</b> == | ||
| - | Glucose-6-Phosphate Dehydrogenase is the rate-limiting enzyme in the pentose phosphate pathway. | + | Glucose-6-Phosphate Dehydrogenase is the rate-limiting enzyme in the pentose phosphate pathway. G6PD converts Glucose-6-Phosphate into 6-phosphoglucono-δ-lactone. Dysfunction of G6PD has multiple down stream effects. The pentose phosphate pathway synthesizes precursors for multiple other biosynthetic pathways. For example, it synthesizes nucleotide precursors for DNA synthesis. |
== <b>Disease </b>== | == <b>Disease </b>== | ||
| Line 16: | Line 16: | ||
Glucose-6-Phosphate Dehydrogenase plays a key role in maintaining homeostasis. Not only does it catalyze the rate limiting step of the oxidative pentose phosphate pathway, it maintains the shape of red blood cells by protecting against potential deadly reactive oxygen species. Glucose-6-Phosphate dehydrogenase deficiency is the most common enzyme deficiency in humans, affecting over 400 million people worldwide. G6PD deficiency resulted in 4100 deaths worldwide in 2013. | Glucose-6-Phosphate Dehydrogenase plays a key role in maintaining homeostasis. Not only does it catalyze the rate limiting step of the oxidative pentose phosphate pathway, it maintains the shape of red blood cells by protecting against potential deadly reactive oxygen species. Glucose-6-Phosphate dehydrogenase deficiency is the most common enzyme deficiency in humans, affecting over 400 million people worldwide. G6PD deficiency resulted in 4100 deaths worldwide in 2013. | ||
== <b>Structural highlights </b> == | == <b>Structural highlights </b> == | ||
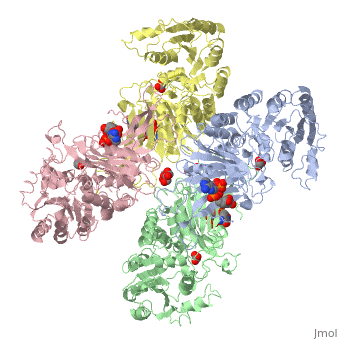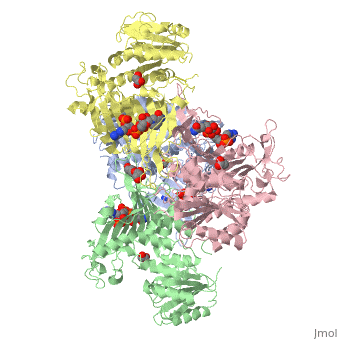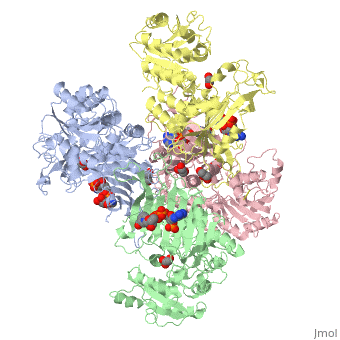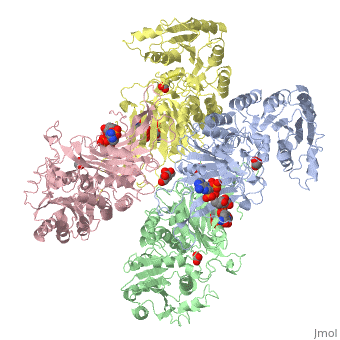
| - | The two dimers are held together in a tetramer by charge-charge interactions. Each dimer has a structural NAD+ | + | The two dimers are held together in a tetramer by charge-charge interactions. Each dimer has a structural NAD+. |
</StructureSection> | </StructureSection> | ||
== <b>References</b> == | == <b>References</b> == | ||
Revision as of 20:08, 20 April 2017
| This Sandbox is Reserved from Jan 17 through June 31, 2017 for use in the course Biochemistry II taught by Jason Telford at the Maryville University, St. Louis, USA. This reservation includes Sandbox Reserved 1225 through Sandbox Reserved 1244. |
To get started:
More help: Help:Editing |
Structure
| |||||||||||
References
Au, Shannon et al. "Human Glucose-6-Phosphate Dehydrogenase: The Crystal Structure Reveals A Structural NADP+ Molecule And Provides Insights Into Enzyme
Deficiency". N.p., 2017. Web. 20 Apr. 2017.
Berg, Jeremy, John Tymoczko, and Lubert Stryer. "Glucose 6-Phosphate Dehydrogenase Plays A Key Role In Protection Against Reactive Oxygen Species".
Ncbi.nlm.nih.gov. N.p., 2017. Web. 20 Apr. 2017.
"G6PD Gene - Genecards | G6PD Protein | G6PD Antibody". Genecards.org. N.p., 2017. Web. 20 Apr. 2017.
"Glucose-6-Phosphate Dehydrogenase". Wikipedia.org. N.p., 2017. Web. 20 Apr. 2017.
"What Is Hemolytic Anemia? - NHLBI, NIH". Nhlbi.nih.gov. N.p., 2017. Web. 20 Apr. 2017.