We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1072
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
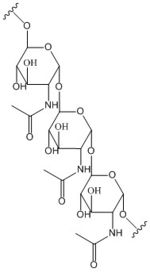
[[Image:Poly B-1, 6 GlcNAc.jpg |150 px|left|thumb|poly-β-1,6-N-acetylglucosamine]] | [[Image:Poly B-1, 6 GlcNAc.jpg |150 px|left|thumb|poly-β-1,6-N-acetylglucosamine]] | ||
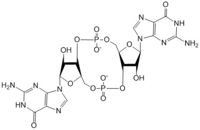
Diguanylate cyclases are a group of class 2 transferase enzymes that catalyze the production of cyclic dimeric-guanosine monophosphate (c-di-GMP), an important <span class="plainlinks">[https://en.wikipedia.org/wiki/Second_messenger_system second messenger]</span> for <span class="plainlinks">[https://en.wikipedia.org/wiki/Signal_transduction signal transduction]</span>. Most commonly through phosphorylation or dephosphoylation events, signal transduction sends messages through cells to promote responses. <span class="plainlinks">[https://en.wikipedia.org/wiki/Escherichia_coli ''Escherechia coli'']</span>, a gram-negative bacterium often found in the intestines of mammals, uses diguanylate cyclase DgcZ in the synthesis of its <span class="plainlinks">[https://en.wikipedia.org/wiki/biofilm biofilm]</span>. Enzyme DgcZ from ''E. coli'' acts as a catalyst to synthesize cyclic di-GMP from two substrate guanosine triphosphate (GTP) molecules to aid in communication of signals throughout the bacteria. C-di-GMP is a second messenger in the production of poly-β-1,6-N-acetylglucosamine (poly-GlcNAc), a polysaccharide required for ''E. coli'' biofilm production. This biofilm allows ''E. coli'' to adhere to extracellular surfaces. The enzyme is only successfully crystallized in its inactive conformation. | Diguanylate cyclases are a group of class 2 transferase enzymes that catalyze the production of cyclic dimeric-guanosine monophosphate (c-di-GMP), an important <span class="plainlinks">[https://en.wikipedia.org/wiki/Second_messenger_system second messenger]</span> for <span class="plainlinks">[https://en.wikipedia.org/wiki/Signal_transduction signal transduction]</span>. Most commonly through phosphorylation or dephosphoylation events, signal transduction sends messages through cells to promote responses. <span class="plainlinks">[https://en.wikipedia.org/wiki/Escherichia_coli ''Escherechia coli'']</span>, a gram-negative bacterium often found in the intestines of mammals, uses diguanylate cyclase DgcZ in the synthesis of its <span class="plainlinks">[https://en.wikipedia.org/wiki/biofilm biofilm]</span>. Enzyme DgcZ from ''E. coli'' acts as a catalyst to synthesize cyclic di-GMP from two substrate guanosine triphosphate (GTP) molecules to aid in communication of signals throughout the bacteria. C-di-GMP is a second messenger in the production of poly-β-1,6-N-acetylglucosamine (poly-GlcNAc), a polysaccharide required for ''E. coli'' biofilm production. This biofilm allows ''E. coli'' to adhere to extracellular surfaces. The enzyme is only successfully crystallized in its inactive conformation. | ||
| + | [[Image:DgcZ_Conformation_change_1.png|250 px|right|thumb|Diagram of DgcZ in its active (left) and inactive (right) conformations. Binding Zinc inactivates the enzyme.]] | ||
== Structural Overview == | == Structural Overview == | ||
[[Image:DgcZ full molecule all sites and ligands labeled.png|250 px|left|thumb|Diguanylate cyclase DgcZ from “E. Coli” with Domains Labeled]] | [[Image:DgcZ full molecule all sites and ligands labeled.png|250 px|left|thumb|Diguanylate cyclase DgcZ from “E. Coli” with Domains Labeled]] | ||
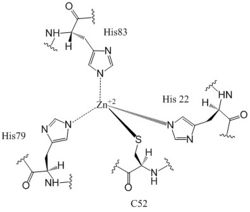
| - | DgcZ is a tetrameric protein from ''E. coli'' made of two domains. Each domain is a dimer, so the whole protein can be called a dimer of dimers. The DgcZ protein has <scene name='69/694239/C2_symmetry/6'>C2</scene> symmetry down its central axis. The catalytic glycine-glycine-glutamate-glutamate-phenylalanine (GGEEF) domain is responsible for synthesizing c-di-GMP, and the regulatory chemoreceptor zinc binding (CZB) domain | + | DgcZ is a tetrameric protein from ''E. coli'' made of two domains. Each domain is a dimer, so the whole protein can be called a dimer of dimers. The DgcZ protein has <scene name='69/694239/C2_symmetry/6'>C2</scene> symmetry down its central axis. The catalytic glycine-glycine-glutamate-glutamate-phenylalanine (GGEEF) domain is responsible for synthesizing c-di-GMP, and the regulatory chemoreceptor zinc binding (CZB) domain houses two zinc binding sites. DgcZ binds zinc in the CZB domain with sub-femtomolar (10<sup>-16</sup>M) affinity. When zinc is bound, the CZB and GGEEF domains adopt conformations that inhibit DgcZ function <sup>[1]</sup>. Enzyme DgcZ was co-crystallized with Zinc fixing the structure in its inactivate conformation. The CZB domain is common to many bacterial lineages, including its prevalence in DgcZ homologs. The domain has an important role in signal transduction of bacteria. CZB and GGEEF domains are prevalent in many bacterial proteins from differing strands of ''E. coli'' <sup>[2]</sup>. The GGEEF domain is catalytic in that it contains the active sites used for cyclizing GTP into c-di-GMP. The CZB domain is used for ligand-mediated regulation of c-di-GMP production. |
===Catalytic GGEEF Domain=== | ===Catalytic GGEEF Domain=== | ||
Revision as of 01:48, 21 April 2017
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Diguanylate Cyclase DgcZ from Escherichia coli
| |||||||||||