We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1053
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
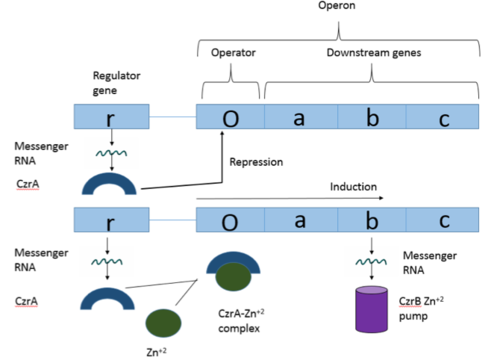
[https://en.wikipedia.org/wiki/Operon Operons] are a critical genetic component of most prokaryotic cells. There are many different operons that are responsible for the production of proteins with a wide range of functions, the most well-known of which are the Lac and Trp operons, responsible for producing enzymes which metabolize lactose and tryptophan respectively. Despite many differences in each operon and the proteins that they encode, operons all function in the same general manner. Structurally, each operon contains a regulator, an operator, and one or more structural genes. The regulator protein is responsible for managing the expression level of the structural genes, the operator is similar to a promoter in a regular gene and is where transcription begins, and the structural genes code for proteins. The regulator protein (produced as a result of expression of the regulator gene) most often acts in a repressive manner, though this is not always the case. That is, the regulator protein will bind to the operator of the operon, inhibiting the binding and/or progression of [https://en.wikipedia.org/wiki/RNA_polymerase RNA polymerase] to the structural genes, thus inhibiting transcription of the genes into mRNA. If the regulator protein were to consistently be active, there could never be adequate expression of the structural genes, so there must be a way to inactive the regulator protein, thus enabling expression of the structural genes. This is achieved through the binding of an inhibitor to the regulator protein. Since regulator proteins are DNA binding proteins, often this inhibition is allosteric rather than competitive, that is the inhibitor is not something that mimics DNA and binds to the active site physically blocking DNA from binding. Rather, the inhibitor of the regulator binds to somewhere other than the active site of the protein, changing it in some way which decreases the proteins affinity or ability to bind DNA. | [https://en.wikipedia.org/wiki/Operon Operons] are a critical genetic component of most prokaryotic cells. There are many different operons that are responsible for the production of proteins with a wide range of functions, the most well-known of which are the Lac and Trp operons, responsible for producing enzymes which metabolize lactose and tryptophan respectively. Despite many differences in each operon and the proteins that they encode, operons all function in the same general manner. Structurally, each operon contains a regulator, an operator, and one or more structural genes. The regulator protein is responsible for managing the expression level of the structural genes, the operator is similar to a promoter in a regular gene and is where transcription begins, and the structural genes code for proteins. The regulator protein (produced as a result of expression of the regulator gene) most often acts in a repressive manner, though this is not always the case. That is, the regulator protein will bind to the operator of the operon, inhibiting the binding and/or progression of [https://en.wikipedia.org/wiki/RNA_polymerase RNA polymerase] to the structural genes, thus inhibiting transcription of the genes into mRNA. If the regulator protein were to consistently be active, there could never be adequate expression of the structural genes, so there must be a way to inactive the regulator protein, thus enabling expression of the structural genes. This is achieved through the binding of an inhibitor to the regulator protein. Since regulator proteins are DNA binding proteins, often this inhibition is allosteric rather than competitive, that is the inhibitor is not something that mimics DNA and binds to the active site physically blocking DNA from binding. Rather, the inhibitor of the regulator binds to somewhere other than the active site of the protein, changing it in some way which decreases the proteins affinity or ability to bind DNA. | ||
[[Image:Operon.png|500px|thumb|center|Figure 1:Overview of Operon Structure]] | [[Image:Operon.png|500px|thumb|center|Figure 1:Overview of Operon Structure]] | ||
| + | |||
| + | == Structure Testing Area == | ||
| + | <Structure load='CzrAwithDNA.pdb' size='350' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | ||
| + | |||
===Czr Operon=== | ===Czr Operon=== | ||
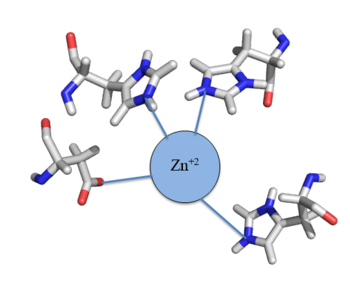
The Chromosome Determined Zinc Responsible (Czr) operon acts as described above, with Czr A acting as the regulator protein. As such, Czr A is responsible for controlling the transcription of the rest of the operon and by extension, the transport of Zn <sup>2+</sup> out of the cell; the role of Czr A in the Czr operon is described in further detail as part of the explanation of biological function. In addition to being a component of an operon, Czr A is also considered to be a metal sensor protein. While the immediate function of Czr A is gene regulation, this serves the larger purpose of acting to maintain an appropriate concentration of Zn <sup>2+</sup> in the cell. | The Chromosome Determined Zinc Responsible (Czr) operon acts as described above, with Czr A acting as the regulator protein. As such, Czr A is responsible for controlling the transcription of the rest of the operon and by extension, the transport of Zn <sup>2+</sup> out of the cell; the role of Czr A in the Czr operon is described in further detail as part of the explanation of biological function. In addition to being a component of an operon, Czr A is also considered to be a metal sensor protein. While the immediate function of Czr A is gene regulation, this serves the larger purpose of acting to maintain an appropriate concentration of Zn <sup>2+</sup> in the cell. | ||
| Line 23: | Line 27: | ||
== Binding of DNA == | == Binding of DNA == | ||
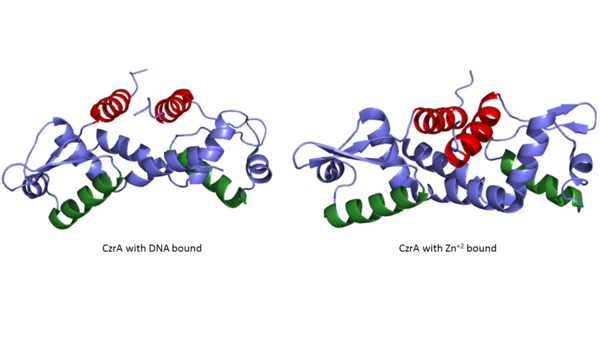
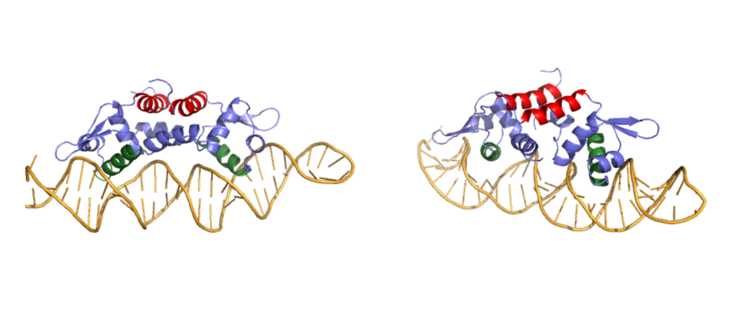
| - | The <scene name='69/694219/Serandhisresidues/3'>main DNA interactions</scene> have been found to occur at the Ser 54 and 57 along with His 58 residues. <scene name='69/694220/2kjb_dna_alpha_4_helix/1'>These residues</scene> are likely to interact with the 5'-TGAA sequence found in the half-site of the DNA. These residues are found in the N terminal of the alpha 4 helix (figure 3). The residues involved in the <scene name='69/694219/Dna_binding_pocket/1'>DNA binding pocket</scene> are Val 42 and Gln 53. This was experimentally determined by mutating the Gln and Val with Ala residues and measuring the binding capacity; In a previously published article <ref name="critical"/>, the DNA bound state of CzrA was tested by using the known critical residues for DNA interactions. <scene name='69/694220/Dna_binding_experiment/1'>Critical DNA binding residues</scene>, Gln53, Val42 (both shown in red), Ser54, Ser57, and His58 (all shown in orange), were replaced with Ala and then compared to the kinetics of the wild type protein. Replacing only the Q53 and V42 residues resulted in an 11-fold and 160-fold decrease in K<sub>a</sub>, respectively. Other residues such as S54, S57, and H58 were also replaced with Ala residues, and it was found that these mutations caused binding similar to the <scene name='69/694220/Dna_residues_when_inhibited/1'>fully inhibited Zn<sup>2+</sup> bound state</scene>. The conformational change that occurs from the Zinc to DNA bound state regarding these residues is small, but the alpha 4 helix (shown in green in Figure 2) does subtly move. Because no major physical change occurs between these two states, it further supports that this region is the main DNA interaction site because of the loss of affinity after the mutation took place. Table 1 in this same article shows the different K<sub>observed</sub>, and the measured decrease in K<sub>observed</sub> for each mutation. The bind between the DNA and the protein can be attributed to losing certain intermolecular forces such as possible hydrogen bonding when changing from Gln and Ala, and a loss of London Dispersion forces in the Val to Ala change. | + | The <scene name='69/694219/Serandhisresidues/3'>main DNA interactions</scene> have been found to occur at the Ser 54 and 57 along with His 58 residues. <scene name='69/694220/2kjb_dna_alpha_4_helix/1'>These residues</scene> are likely to interact with the 5'-TGAA sequence found in the half-site of the DNA. The alpha 4 helices <scene name='69/694219/Czra_with_dna/2'>form an interaction with DNA</scene>. These residues are found in the N terminal of the alpha 4 helix (figure 3). The residues involved in the <scene name='69/694219/Dna_binding_pocket/1'>DNA binding pocket</scene> are Val 42 and Gln 53. This was experimentally determined by mutating the Gln and Val with Ala residues and measuring the binding capacity; In a previously published article <ref name="critical"/>, the DNA bound state of CzrA was tested by using the known critical residues for DNA interactions. <scene name='69/694220/Dna_binding_experiment/1'>Critical DNA binding residues</scene>, Gln53, Val42 (both shown in red), Ser54, Ser57, and His58 (all shown in orange), were replaced with Ala and then compared to the kinetics of the wild type protein. Replacing only the Q53 and V42 residues resulted in an 11-fold and 160-fold decrease in K<sub>a</sub>, respectively. Other residues such as S54, S57, and H58 were also replaced with Ala residues, and it was found that these mutations caused binding similar to the <scene name='69/694220/Dna_residues_when_inhibited/1'>fully inhibited Zn<sup>2+</sup> bound state</scene>. The conformational change that occurs from the Zinc to DNA bound state regarding these residues is small, but the alpha 4 helix (shown in green in Figure 2) does subtly move. Because no major physical change occurs between these two states, it further supports that this region is the main DNA interaction site because of the loss of affinity after the mutation took place. Table 1 in this same article shows the different K<sub>observed</sub>, and the measured decrease in K<sub>observed</sub> for each mutation. The bind between the DNA and the protein can be attributed to losing certain intermolecular forces such as possible hydrogen bonding when changing from Gln and Ala, and a loss of London Dispersion forces in the Val to Ala change. |
The differences in binding favorability can also be seen when comparing the ΔG for the Apo-state vs. the DNA bound state and the Zinc vs. the Zinc and DNA bound state. These ΔGs were found to be -15.2kcal/mol and -9kcal/mol respectively<ref>DOI: 10.1021/ja208047b</ref>. This agrees with previously published data showing the Zinc binding inhibits the affinity the protein has to DNA. | The differences in binding favorability can also be seen when comparing the ΔG for the Apo-state vs. the DNA bound state and the Zinc vs. the Zinc and DNA bound state. These ΔGs were found to be -15.2kcal/mol and -9kcal/mol respectively<ref>DOI: 10.1021/ja208047b</ref>. This agrees with previously published data showing the Zinc binding inhibits the affinity the protein has to DNA. | ||
Revision as of 17:40, 18 July 2017
CzrA: A Zinc Dependent Transcriptional Regulator
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Arunkumar A., Campanello G., Giedroc D. (2009). Solution Structure of a paradigm ArsR family zinc sensor in the DNA-bound state. PNAS 106:43 18177-18182.
- ↑ Chakravorty DK, Wang B, Lee CW, Giedroc DP, Merz KM Jr. Simulations of allosteric motions in the zinc sensor CzrA. J Am Chem Soc. 2012 Feb 22;134(7):3367-76. doi: 10.1021/ja208047b. Epub 2011 Nov , 14. PMID:22007899 doi:http://dx.doi.org/10.1021/ja208047b
- ↑ MacPherson S, Larochelle M, Turcotte B. A fungal family of transcriptional regulators: the zinc cluster proteins. Microbiol Mol Biol Rev. 2006 Sep;70(3):583-604. PMID:16959962 doi:http://dx.doi.org/10.1128/MMBR.00015-06
- ↑ Miller J, McLachlan AD, Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun 4;4(6):1609-1614.
- ↑ Grossoehme NE, Giedroc DP. Energetics of allosteric negative coupling in the zinc sensor S. aureus CzrA. J Am Chem Soc. 2009 Dec 16;131(49):17860-70. doi: 10.1021/ja906131b. PMID:19995076 doi:http://dx.doi.org/10.1021/ja906131b