Sandbox Reserved 1063
From Proteopedia
(Difference between revisions)
| Line 28: | Line 28: | ||
=== Hydrogen Bond Network === | === Hydrogen Bond Network === | ||
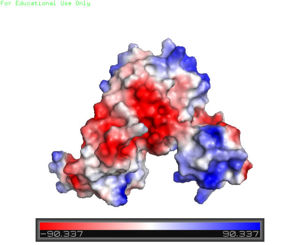
| - | [[Image:Charge_map.jpg |300 px|right|thumb|'''Figure 3'''. A charge map of AdcR shows the general triangular shape and the <font color='blue'>positively</font> charged area on the tips of the wHTH | + | [[Image:Charge_map.jpg |300 px|right|thumb|'''Figure 3'''. A charge map of AdcR shows the general triangular shape and the <font color='blue'>positively</font> charged area on the tips of the wHTH motif]] |
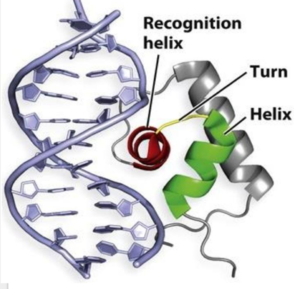
The binding of zinc metals creates a hydrogen bond network within the protein that connects the metal binding sites and the [https://en.wikipedia.org/wiki/DNA-binding_domain DNA binding domain]. The <scene name='69/694230/Hydrogen_bonding_1/5'>hydrogen bond network</scene> (<scene name='69/694230/Hydrogen_bonding_2/4'>with measurements</scene>) is represented by each atom type in the 3D model. The hydrogen bond network is characteristic of the MarR family as a whole. More importantly, the hydrogen bonding network connects the metal binding pockets to the α4 helix also known as the recognition helix. <scene name='69/694230/Recognition_helix/3'>Specific residues</scene> in the recognition helix recognize a sequence of DNA that is unknown at the moment; however, scientists do know that the hydrogen bond network acts as an allosteric activator for the protein to bind DNA. The hydrogen bond network connects the α2 and α4 helix via hydrogen bonding between specific residues. After zinc is bound, a glutamate (E24) residue from a random coil accepts a hydrogen bond from the carboxamide end of an asparagine (N38) residue from the α2 helix. Then, a glutamine (Q40) residue from α2 helix accepts a hydrogen bond from a serine (S74) residue from the α4 helix <ref name="guerra" />. The binding of zinc allows for these conformational changes that induces the binding of DNA in order to activate genes. | The binding of zinc metals creates a hydrogen bond network within the protein that connects the metal binding sites and the [https://en.wikipedia.org/wiki/DNA-binding_domain DNA binding domain]. The <scene name='69/694230/Hydrogen_bonding_1/5'>hydrogen bond network</scene> (<scene name='69/694230/Hydrogen_bonding_2/4'>with measurements</scene>) is represented by each atom type in the 3D model. The hydrogen bond network is characteristic of the MarR family as a whole. More importantly, the hydrogen bonding network connects the metal binding pockets to the α4 helix also known as the recognition helix. <scene name='69/694230/Recognition_helix/3'>Specific residues</scene> in the recognition helix recognize a sequence of DNA that is unknown at the moment; however, scientists do know that the hydrogen bond network acts as an allosteric activator for the protein to bind DNA. The hydrogen bond network connects the α2 and α4 helix via hydrogen bonding between specific residues. After zinc is bound, a glutamate (E24) residue from a random coil accepts a hydrogen bond from the carboxamide end of an asparagine (N38) residue from the α2 helix. Then, a glutamine (Q40) residue from α2 helix accepts a hydrogen bond from a serine (S74) residue from the α4 helix <ref name="guerra" />. The binding of zinc allows for these conformational changes that induces the binding of DNA in order to activate genes. | ||
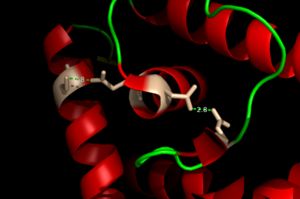
[[Image:H Bonding of DNA.png|300 px|left|thumb|'''Figure 4'''. The Hydrogen Bonding Network is shown with dotted green lines approximately 2.8 angstroms between residues.]] | [[Image:H Bonding of DNA.png|300 px|left|thumb|'''Figure 4'''. The Hydrogen Bonding Network is shown with dotted green lines approximately 2.8 angstroms between residues.]] | ||
Revision as of 19:30, 21 April 2017
Adhesin Competence Regulator
| |||||||||||
References
- ↑ Sanson M, Makthal N, Flores AR, Olsen RJ, Musser JM, Kumaraswami M. Adhesin competence repressor (AdcR) from Streptococcus pyogenes controls adaptive responses to zinc limitation and contributes to virulence. Nucleic Acids Res. 2015 Jan;43(1):418-32. doi: 10.1093/nar/gku1304. Epub 2014 Dec, 15. PMID:25510500 doi:http://dx.doi.org/10.1093/nar/gku1304
- ↑ Ma Z, Jacobsen FE, Giedroc DP. Coordination chemistry of bacterial metal transport and sensing. Chem Rev. 2009 Oct;109(10):4644-81. doi: 10.1021/cr900077w. PMID:19788177 doi:http://dx.doi.org/10.1021/cr900077w
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Guerra AJ, Dann CE, Giedroc DP. Crystal Structure of the Zinc-Dependent MarR Family Transcriptional Regulator AdcR in the Zn(II)-Bound State. J Am Chem Soc. 2011 Nov 21. PMID:22085181 doi:10.1021/ja2080532
- ↑ 4.0 4.1 Reyes-Caballero H, Guerra AJ, Jacobsen FE, Kazmierczak KM, Cowart D, Koppolu UM, Scott RA, Winkler ME, Giedroc DP. The metalloregulatory zinc site in Streptococcus pneumoniae AdcR, a zinc-activated MarR family repressor. J Mol Biol. 2010 Oct 22;403(2):197-216. doi: 10.1016/j.jmb.2010.08.030. Epub 2010, Sep 8. PMID:20804771 doi:http://dx.doi.org/10.1016/j.jmb.2010.08.030