Sandbox Reserved 1236
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
Luciferase is a class of bioluminescent enzymes that are found in several luminescence organisms. The most studied form Luciferase within its class is found in the North American Firefly (Photinus pyralis). This protein catalyzed the reaction that produces the distinctive yellow flash seen in from the abdomen of the insect. Photinus pyralis is known to use this mechanism for mate attraction and defense. Firefly Luciferase is unique to its species and different forms within the luciferase class can be found in other invertebrates and bacteria.<ref>Conti, E., Franks, N. P., & Brick, P. (1996). Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes. Structure, 4(3), 287-298.</ref>. The most notable being the bioluminescent enzyme found in Click Beetles (Pyrophorus). This protein is one of the most studied and widely used luminescence enzymes having various applications in cell and molecular biology. | Luciferase is a class of bioluminescent enzymes that are found in several luminescence organisms. The most studied form Luciferase within its class is found in the North American Firefly (Photinus pyralis). This protein catalyzed the reaction that produces the distinctive yellow flash seen in from the abdomen of the insect. Photinus pyralis is known to use this mechanism for mate attraction and defense. Firefly Luciferase is unique to its species and different forms within the luciferase class can be found in other invertebrates and bacteria.<ref>Conti, E., Franks, N. P., & Brick, P. (1996). Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes. Structure, 4(3), 287-298.</ref>. The most notable being the bioluminescent enzyme found in Click Beetles (Pyrophorus). This protein is one of the most studied and widely used luminescence enzymes having various applications in cell and molecular biology. | ||
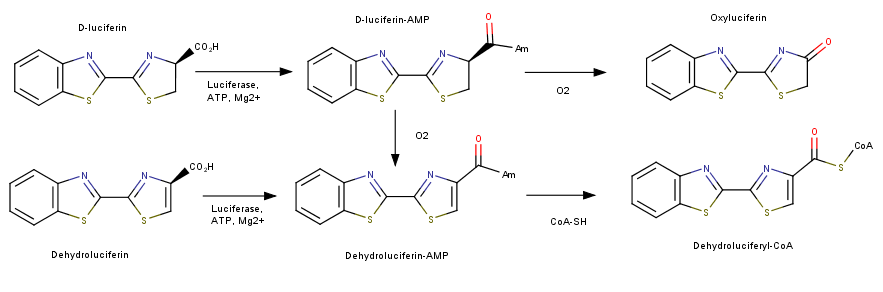
| - | Luciferase catalyzes two reactions; both reactions use an adenylation reaction along with ATP. The bioluminescent pathway produces Oxyluciferin and a photon of light. The other pathways does not produce light and is known as the nonluminescent reaction. This mechanism utilizes luciferin and produces Luciferyl-CoA. This addiotion of a CoA has been shown to be related to Acyl-CoA synthase used in the activation of fatty acids for oxidation. | + | Luciferase catalyzes two reactions; both reactions use an adenylation reaction along with ATP. The bioluminescent pathway produces Oxyluciferin and a photon of light. The other pathways does not produce light and is known as the nonluminescent reaction. This mechanism utilizes luciferin and produces Luciferyl-CoA. This addiotion of a CoA has been shown to be related to Acyl-CoA synthase used in the activation of fatty acids for oxidation. <ref>Nakamura, Mitsuhiro, Shojiro Maki, Yoshiharu Amano, Yutaka Ohkita, Kazuki Niwa, Takashi Hirano, Yoshihiro Ohmiya, and Haruki Niwa. "Firefly luciferase exhibits bimodal action depending on the luciferin chirality." Biochemical and Biophysical Research Communications 331.2 (2005): 471-75. Web.</ref> |
[[Image:luciferase.png]] | [[Image:luciferase.png]] | ||
Revision as of 22:05, 28 April 2017
| This Sandbox is Reserved from Jan 17 through June 31, 2017 for use in the course Biochemistry II taught by Jason Telford at the Maryville University, St. Louis, USA. This reservation includes Sandbox Reserved 1225 through Sandbox Reserved 1244. |
To get started:
More help: Help:Editing |
Luciferase
| |||||||||||
References
- ↑ Conti, E., Franks, N. P., & Brick, P. (1996). Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes. Structure, 4(3), 287-298.
- ↑ Nakamura, Mitsuhiro, Shojiro Maki, Yoshiharu Amano, Yutaka Ohkita, Kazuki Niwa, Takashi Hirano, Yoshihiro Ohmiya, and Haruki Niwa. "Firefly luciferase exhibits bimodal action depending on the luciferin chirality." Biochemical and Biophysical Research Communications 331.2 (2005): 471-75. Web.
- ↑ Nakamura, Mitsuhiro, Shojiro Maki, Yoshiharu Amano, Yutaka Ohkita, Kazuki Niwa, Takashi Hirano, Yoshihiro Ohmiya, and Haruki Niwa. "Firefly luciferase exhibits bimodal action depending on the luciferin chirality." Biochemical and Biophysical Research Communications 331.2 (2005): 471-75. Web.
- ↑ Khurana, Pankaj, Rajesh S. Gokhale, and Debasisa Mohanty. "Genome scale prediction of substrate specificity for acyl adenylate superfamily of enzymes based on active site residue profiles." BMC Bioinformatics 11.1 (2010): 57. ResearchGate. Web. 28 Mar. 2017.
- ↑ Wet, J. R., Wood, K. V., Helinski, D. R., & Deluca, M. (1985). Cloning of firefly luciferase cDNA and the expression of active luciferase in Escherichia coli. Proceedings of the National Academy of Sciences, 82(23), 7870-7873.
- ↑ Viviani, V. R. (2002). The origin, diversity, and structure function relationships of insect luciferases. Cellular and Molecular Life Sciences, 59(11), 1833-1850.
- ↑ Branchini, B. R., Magyar, R. A., Marcantonio, K. M., Newberry, K. J., Stroh, J. G., Hinz, L. K., & Murtiashaw, M. H. (1997). Identification of a Firefly Luciferase Active Site Peptide Using a Benzophenone-based Photooxidation Reagent. Journal of Biological Chemistry, 272(31), 19359-19364.
- ↑ Goodsell, David . "Molecule of the Month: Luciferase." PDB-101: Luciferase. RSCB Protein Data Bank, June 2006. Web. 28 Apr. 2017.