Luciferase is an enzyme responsible for catalyzation of many bioluminencent pathways found in various organism. It is a highly studied and utilized protein for applications in cell biology, genetics and biochemistry.
Function
Luciferase is a class of bioluminescent enzymes that are found in several luminescence organisms. The most studied form Luciferase within its class is found in the North American Firefly (Photinus pyralis). This protein catalyzed the reaction that produces the distinctive yellow flash seen in from the abdomen of the insect. Photinus pyralis is known to use this mechanism for mate attraction and defense. Firefly Luciferase is unique to its species and different forms within the luciferase class can be found in other invertebrates and bacteria.[1]. The most notable being the bioluminescent enzyme found in Click Beetles (Pyrophorus). This protein is one of the most studied and widely used luminescence enzymes having various applications in cell and molecular biology.
Luciferase catalyzes two reactions; both reactions use an adenylation reaction along with ATP. The bioluminescent pathway produces Oxyluciferin and a photon of light. The other pathways does not produce light and is known as the nonluminescent reaction. This mechanism utilizes luciferin and produces Luciferyl-CoA. This addiotion of a CoA has been shown to be related to Acyl-CoA synthase used in the activation of fatty acids for oxidation. [2]
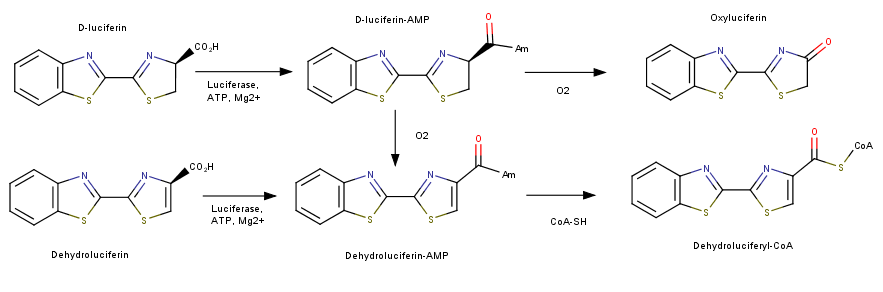
This image depict the two reactions that luciferase can catalyze. The first being the bioluminencent pathway producing a photon of light and the second, following a mechanism similar to fatty acid synthase, producing a Dehydroluciferyl-CoA. [3]
Origin
Luciferase is part of the acyl-adenylate/thioester-forming superfamily. Through its two step reaction it ultimately forms an acyl-adenylate intermediate which is esterified into CoA. This mechanism is similar to the other members in this class some of which include Fatty Acyl AMP Ligases, Fatty Acyl CoA Ligases, and Acetyl CoA Ligases. There is evidence that Luciferase has homology with the highly conserved enzymes used in lipid synthesis. [4] Recent studies show that the Gene sequencing of firefly luciferase along with other related preformed and compared to other homologs of the gene. Studies suggest that firefly luciferase gene has similarities to drosophilla fatty acyl-Coa genes.
Disease
Relevance
This protein has been utilized in various types of assays ranging from quantification of ATP and the rate of transcription within a cell.[5]. This molecule is especially unique due to the fact that is very efficient in producing a photon through this reaction. Luciferase is sensitive to small changes in substrate and is a optimal choice for quantification of gene expression. It has potential for further biological applications in the future. Luciferase is widely used as a luminescent reporter gene in a variety of assays. The Luciferase gene can be isolated from the firefly and be insterted into a plasmid. This plasmid could contain a gene that is destined for transfection into a cell. After transfection luciferin can be added to a cell culture in order to visualize the expression of the transfected gene. The amount of fluorescence can be quantified to determine the amount of expression within a cell. [6]
Structural highlights
The structure of this protein comprises of two prominent domains. The larger one contains an N terminal distorted beta-barrel accompanied by alpha helices. The second and smaller unit is consist of a beta sheet and alpha helix complex [7]. The process of fluorescence is achieved through a two-step oxidation reaction involving the substrate Lucinferin accompanied with ATP, Magnesium and oxygen. The first step consist of using ATP-Mg in an Acylation reaction of the COOH group on Lucinferin producing a Luciferyl adenylate intermediate and a phosphate group. The second reaction uses oxygen to create an excited state of the molecule. The molecule then returns to its ground state emitting a photon of light. [8]
A single peptide has been discover that plays a vital role in the photooxidation by Luciferase. The specific amino acid is a histidine located in the region of the protein [9]. It has been shown to be necessary for the use of oxygen in the second part of the reaction.
Structure Specifications
The attractive feature for the study and application of luciferase is its bioluminescent activity. The color of light produced has been found to vary depending on the organism that the protein is retrieved from. The fluorsence of light ranges through various wavelengths of light some of which include red, green and yellow light. This difference has a connection to the specificity of the active site of luciferase. Depending on the source of the protein different residues will produce a certain color of fluorescence. Variations in luciferase extracted from japanese fire flies has revealed this specificity. In high energy analogs of luciferase reveal the 286th residue is important in determine the color of light. When comparing green fluorescent japanese luciferin the active site contain an Ser286, while the red emitting variant has an Asp286. These findings indicate that slight variations in the active sight of luciferase can produce various effects on its bioluminencent activity. [10]
Inhibitors
Inhibition of luciferase can be mediated by several types of molecules. The presence of CoA has been found to have down regulate the bioluminescencent pathway of luciferase. Increased CoA favors the nonluminescent pathway which does not create a photon of light and instead produces Dehydroluciferyl-CoA. In addition to CoA inhibition of this enzyme has been seen with the exposure to diverse group of anesthetics. These molecules bind allosterically to luciferase and cause a conformation change in the protein. This unfolding alters the active site of the protein enough to not allow the bioluminescent catalysis to occur. Other than macro molecules, certain metal ions, specifically Ni and Co, have been seen to inhibit this reaction by replacing Magnesium in the active site. Magnesium is required for use of ATP and without it the reaction will not take place. Of the many inhibitors identified, lipoic acid demonstrates strong activity. Lipoic acid competes with luciferin for the active site enabling the binding of the substrate for the reaction. [11]
Luciferase in fungi
Luciferase is known to be found in various bacteria and insects, but recent findings suggest that is may also be present in bioluminescent fungi. A similar but unique pathway is currently being studied. With approximately 80 species of bioluminescent fungi known in nature today similar pathways of the luciferase pathway could be discovered in the near future. [12]
References
- ↑ Conti, E., Franks, N. P., & Brick, P. (1996). Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes. Structure, 4(3), 287-298.
- ↑ Nakamura, Mitsuhiro, Shojiro Maki, Yoshiharu Amano, Yutaka Ohkita, Kazuki Niwa, Takashi Hirano, Yoshihiro Ohmiya, and Haruki Niwa. "Firefly luciferase exhibits bimodal action depending on the luciferin chirality." Biochemical and Biophysical Research Communications 331.2 (2005): 471-75. Web.
- ↑ Nakamura, Mitsuhiro, Shojiro Maki, Yoshiharu Amano, Yutaka Ohkita, Kazuki Niwa, Takashi Hirano, Yoshihiro Ohmiya, and Haruki Niwa. "Firefly luciferase exhibits bimodal action depending on the luciferin chirality." Biochemical and Biophysical Research Communications 331.2 (2005): 471-75. Web.
- ↑ Khurana, Pankaj, Rajesh S. Gokhale, and Debasisa Mohanty. "Genome scale prediction of substrate specificity for acyl adenylate superfamily of enzymes based on active site residue profiles." BMC Bioinformatics 11.1 (2010): 57. ResearchGate. Web. 28 Mar. 2017.
- ↑ Wet, J. R., Wood, K. V., Helinski, D. R., & Deluca, M. (1985). Cloning of firefly luciferase cDNA and the expression of active luciferase in Escherichia coli. Proceedings of the National Academy of Sciences, 82(23), 7870-7873.
- ↑ "Luciferase Assays." Thermo Fisher Scientific. Thermo Fisher Scientific, 2017. Web. 28 Apr. 2017.
- ↑ Viviani, V. R. (2002). The origin, diversity, and structure function relationships of insect luciferases. Cellular and Molecular Life Sciences, 59(11), 1833-1850.
- ↑ Conti, E., Franks, N. P., & Brick, P. (1996). Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes. Structure, 4(3), 287-298.
- ↑ Branchini, B. R., Magyar, R. A., Marcantonio, K. M., Newberry, K. J., Stroh, J. G., Hinz, L. K., & Murtiashaw, M. H. (1997). Identification of a Firefly Luciferase Active Site Peptide Using a Benzophenone-based Photooxidation Reagent. Journal of Biological Chemistry, 272(31), 19359-19364.
- ↑ Goodsell, David . "Molecule of the Month: Luciferase." PDB-101: Luciferase. RSCB Protein Data Bank, June 2006. Web. 28 Apr. 2017.
- ↑ Leitão, João M. M., and Joaquim C G Esteves Da Silva. "Firefly luciferase inhibition." Journal of photochemistry and photobiology. (2010): 1-8. Research Gate. Web. 28 Apr. 2017.
- ↑ American Association for the Advancement of Science. "Illuminating the secret of glow-in-the-dark mushrooms: Mechanism and color modulation of fungal bioluminescence." ScienceDaily. ScienceDaily, 26 April 2017.

