We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Rhodopsin
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
===Rhodopsin Architecture=== | ===Rhodopsin Architecture=== | ||
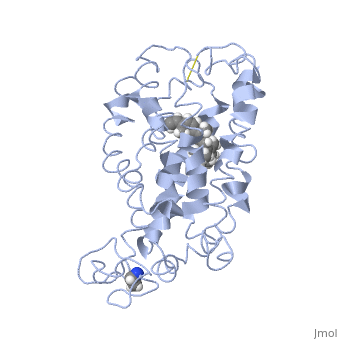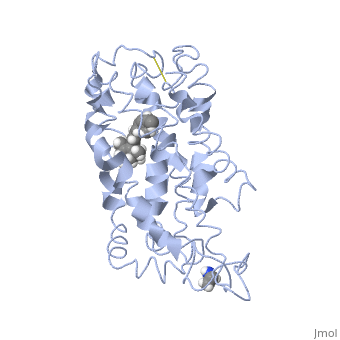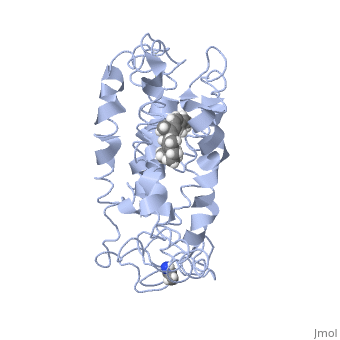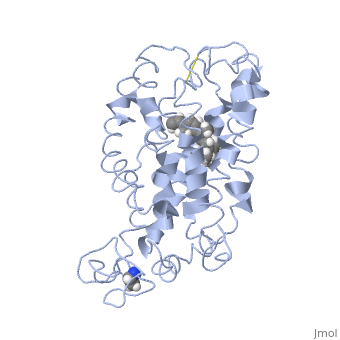
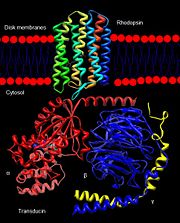
Rhodopsin consists of seven mostly α-helical transmembrane domains (H1-H7) linked sequentially by extracellular and cytoplasmic loops (E1-E3 and C1-C3 respectively), with the extracellular amino-terminal tail and the cytoplasmic carboxyl-terminal tail<ref name="Article12"/>. Four of the helices are tilted and three of the helices are approximately perpendicular to the membrane plane<ref name="Article4">PMID:9199406</ref>. There is notable interaction between the four extracellular domains, but only a few associations are observed with the cytoplasmic domains<ref name="Article9">PMID:11343925</ref>. Helix 7 is close to being elongated around the Lysine 296 retinal attachment site, and also contains the residues Proline 291 and Proline 303, with Proline 303 being part of a conserved motif<ref name="Article9"/>. Near the retinal region, there is a <scene name='40/400594/Cv/2'>β4 strand (Serine 186-Cysteine 187-Glycine 188-Isoleucine 189)</scene> within the Extracellular Helix 2 that runs almost parallel to the chromophore held in place and is stabilized by the essential conserved | Rhodopsin consists of seven mostly α-helical transmembrane domains (H1-H7) linked sequentially by extracellular and cytoplasmic loops (E1-E3 and C1-C3 respectively), with the extracellular amino-terminal tail and the cytoplasmic carboxyl-terminal tail<ref name="Article12"/>. Four of the helices are tilted and three of the helices are approximately perpendicular to the membrane plane<ref name="Article4">PMID:9199406</ref>. There is notable interaction between the four extracellular domains, but only a few associations are observed with the cytoplasmic domains<ref name="Article9">PMID:11343925</ref>. Helix 7 is close to being elongated around the Lysine 296 retinal attachment site, and also contains the residues Proline 291 and Proline 303, with Proline 303 being part of a conserved motif<ref name="Article9"/>. Near the retinal region, there is a <scene name='40/400594/Cv/2'>β4 strand (Serine 186-Cysteine 187-Glycine 188-Isoleucine 189)</scene> within the Extracellular Helix 2 that runs almost parallel to the chromophore held in place and is stabilized by the essential conserved | ||
| - | <scene name=' | + | <scene name='40/400594/Cv/3'>disulfide bond between Cysteine 110 and Cysteine 187</scene>. This loop also potentially contacts the chromophore through Glutamine 181 and Tyrosine 191<ref name="Article12"/>. |
<scene name='Sandbox_173/Water_molecules/1'>Water molecules</scene> are observed to be located in the extracellular domains of rhodopsin; specifically, the water molecules around the second extracellular loop between Helix 4 and 5 solvate the loop when the loop interacts with the retinal chromophore and possibly contribute to its flexibility should rearrangement occur<ref name="ReferenceArticle">PMID:15327956</ref>. | <scene name='Sandbox_173/Water_molecules/1'>Water molecules</scene> are observed to be located in the extracellular domains of rhodopsin; specifically, the water molecules around the second extracellular loop between Helix 4 and 5 solvate the loop when the loop interacts with the retinal chromophore and possibly contribute to its flexibility should rearrangement occur<ref name="ReferenceArticle">PMID:15327956</ref>. | ||
Revision as of 09:14, 10 May 2017
| |||||||||||
3D structures of rhodopsin
Updated on 10-May-2017
References
- ↑ Hornak V, Ahuja S, Eilers M, Goncalves JA, Sheves M, Reeves PJ, Smith SO. Light activation of rhodopsin: insights from molecular dynamics simulations guided by solid-state NMR distance restraints. J Mol Biol. 2010 Feb 26;396(3):510-27. Epub 2009 Dec 11. PMID:20004206 doi:10.1016/j.jmb.2009.12.003
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Sakmar TP. Structure of rhodopsin and the superfamily of seven-helical receptors: the same and not the same. Curr Opin Cell Biol. 2002 Apr;14(2):189-95. PMID:11891118
- ↑ 3.0 3.1 3.2 3.3 Kristiansen K. Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol Ther. 2004 Jul;103(1):21-80. PMID:15251227 doi:10.1016/j.pharmthera.2004.05.002
- ↑ Millar RP, Newton CL. The year in G protein-coupled receptor research. Mol Endocrinol. 2010 Jan;24(1):261-74. Epub 2009 Dec 17. PMID:20019124 doi:10.1210/me.2009-0473
- ↑ 5.0 5.1 5.2 Meng EC, Bourne HR. Receptor activation: what does the rhodopsin structure tell us? Trends Pharmacol Sci. 2001 Nov;22(11):587-93. PMID:11698103
- ↑ 6.0 6.1 Shieh T, Han M, Sakmar TP, Smith SO. The steric trigger in rhodopsin activation. J Mol Biol. 1997 Jun 13;269(3):373-84. PMID:9199406 doi:10.1006/jmbi.1997.1035
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 Okada T, Ernst OP, Palczewski K, Hofmann KP. Activation of rhodopsin: new insights from structural and biochemical studies. Trends Biochem Sci. 2001 May;26(5):318-24. PMID:11343925
- ↑ 8.0 8.1 Okada T, Sugihara M, Bondar AN, Elstner M, Entel P, Buss V. The retinal conformation and its environment in rhodopsin in light of a new 2.2 A crystal structure. J Mol Biol. 2004 Sep 10;342(2):571-83. PMID:15327956 doi:10.1016/j.jmb.2004.07.044
- ↑ 9.0 9.1 Janz JM, Farrens DL. Assessing structural elements that influence Schiff base stability: mutants E113Q and D190N destabilize rhodopsin through different mechanisms. Vision Res. 2003 Dec;43(28):2991-3002. PMID:14611935
- ↑ 10.0 10.1 10.2 Kisselev OG. Focus on molecules: rhodopsin. Exp Eye Res. 2005 Oct;81(4):366-7. PMID:16051215 doi:10.1016/j.exer.2005.06.018
- ↑ 11.0 11.1 11.2 Verhoeven MA, Bovee-Geurts PH, de Groot HJ, Lugtenburg J, DeGrip WJ. Methyl substituents at the 11 or 12 position of retinal profoundly and differentially affect photochemistry and signalling activity of rhodopsin. J Mol Biol. 2006 Oct 13;363(1):98-113. Epub 2006 Jul 28. PMID:16962138 doi:10.1016/j.jmb.2006.07.039
- ↑ 12.0 12.1 12.2 12.3 Morris MB, Dastmalchi S, Church WB. Rhodopsin: structure, signal transduction and oligomerisation. Int J Biochem Cell Biol. 2009 Apr;41(4):721-4. Epub 2008 Aug 3. PMID:18692154 doi:10.1016/j.biocel.2008.04.025
- ↑ 13.0 13.1 13.2 13.3 13.4 Nelson, D., and Cox, M. Lehninger Principles of Biochemistry. 2008. 5th edition. W. H. Freeman and Company, New York, New York, USA. pp. 462-465.
- ↑ Hurley JB, Spencer M, Niemi GA. Rhodopsin phosphorylation and its role in photoreceptor function. Vision Res. 1998 May;38(10):1341-52. PMID:9667002
- ↑ 15.0 15.1 15.2 Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature. 2008 Jul 10;454(7201):183-7. Epub 2008 Jun 18. PMID:18563085 doi:10.1038/nature07063
- ↑ 16.0 16.1 Surya A, Knox BE. Enhancement of opsin activity by all-trans-retinal. Exp Eye Res. 1998 May;66(5):599-603. PMID:9628807 doi:10.1006/exer.1997.0453
See Also
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Wayne Decatur, Jaime Prilusky, Joel L. Sussman, Cinting Lim