This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sentrin-specific protease
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
| - | '''Sentrin-specific protease''' 1 (Senp1), Senp2, Senp7, Senp8 catalyze the processing of SUM1, SUMO2 and SUMO3 to their mature forms and deconjugate these proteins from their target proteins. Senp’s hydrolyze a peptide bond in the C-terminal of SUMO propeptide<ref>PMID:24586832</ref>. | + | '''Sentrin-specific protease''' 1 (Senp1), Senp2, Senp7, Senp8 catalyze the processing of SUM1, SUMO2 and SUMO3 to their mature forms and deconjugate these proteins from their target proteins. Senp’s hydrolyze a peptide bond in the C-terminal of SUMO propeptide<ref>PMID:24586832</ref>.<br /> |
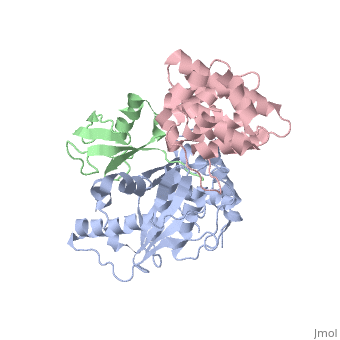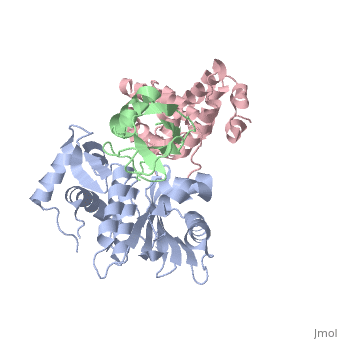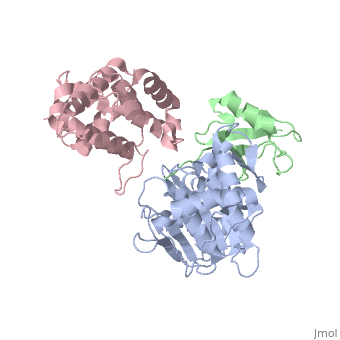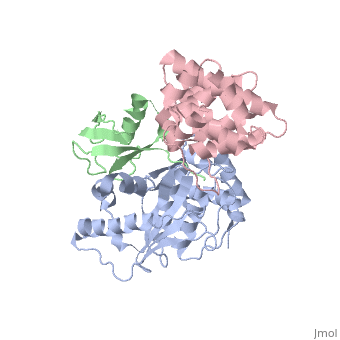
<scene name='56/568937/Cv/1'>human Senp1 catalytic domain complex with SUMO1 and RanGAP C terminal</scene> (PDB entry [[2iy0]]). | <scene name='56/568937/Cv/1'>human Senp1 catalytic domain complex with SUMO1 and RanGAP C terminal</scene> (PDB entry [[2iy0]]). | ||
| Line 22: | Line 22: | ||
*Sentrin-specific protease 2 | *Sentrin-specific protease 2 | ||
| - | **[[1tgz]] – hSenp2 catalytic domain + SUMO1<br /> | ||
**[[1th0]] – hSenp2 catalytic domain <br /> | **[[1th0]] – hSenp2 catalytic domain <br /> | ||
| + | **[[1tgz]] – hSenp2 catalytic domain + SUMO1<br /> | ||
**[[2io0]], [[5aek]] – hSenp2 catalytic domain (mutant) + SUMO1 precursor<br /> | **[[2io0]], [[5aek]] – hSenp2 catalytic domain (mutant) + SUMO1 precursor<br /> | ||
**[[3zo5]] - hSenp2 catalytic domain (mutant) + SUMO2 precursor<br /> | **[[3zo5]] - hSenp2 catalytic domain (mutant) + SUMO2 precursor<br /> | ||
Revision as of 19:24, 17 September 2018
| |||||||||||
3D structures of sentrin-specific protease
Updated on 17-September-2018
References
- ↑ Lee WP, Jena S, Doherty D, Ventakesh J, Schimdt J, Furmick J, Widener T, Lemau J, Jurutka PW, Thompson PD. Sentrin/SUMO specific proteases as novel tissue-selective modulators of vitamin D receptor-mediated signaling. PLoS One. 2014 Feb 20;9(2):e89506. doi: 10.1371/journal.pone.0089506. eCollection, 2014. PMID:24586832 doi:http://dx.doi.org/10.1371/journal.pone.0089506