Jumonji domain-containing protein
From Proteopedia
| Line 1: | Line 1: | ||
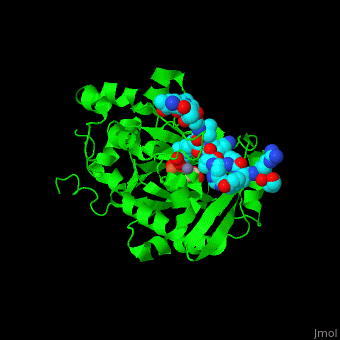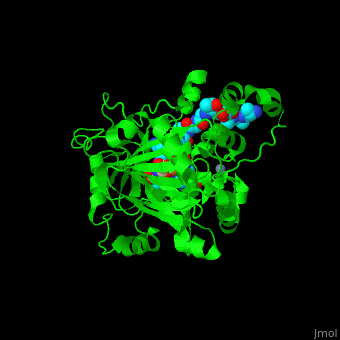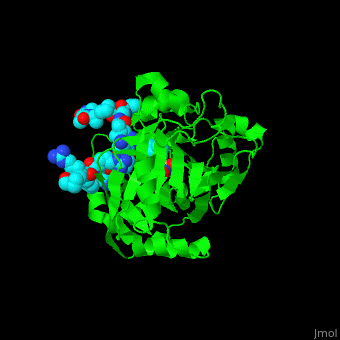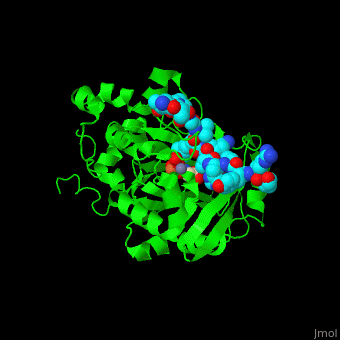
| - | <StructureSection load='2p5b' size=' | + | <StructureSection load='2p5b' size='350' side='right' caption='Structure of human Jumonji domain-containing protein 2a catalytic domain complex with oxalylglycine, Fe+2 ion, Zn+2 ion (grey) and histone H3 peptide (aqua) with trimethyllysine (magenta) (PDB code [[2p5b]]).' scene='59/596434/Cv/1'> |
SEE ALSO [[Lysine-specific histone demethylase]] | SEE ALSO [[Lysine-specific histone demethylase]] | ||
== Function == | == Function == | ||
| - | *'''Jumonji domain-containing protein 2a''' (Jmjd2a) or '''KDM4A''' catalyzes the demethylation of trimethylated histone H3 at lysine residues 9 and 36 producing the dimethylated form. Jmjd2a domains contain: N-terminal, catalytic core (residues 1-350), 2 TUDOR domains which recognize dimethylated Arg (residues 895-1011) , 2 PHD-type zinc fingers and C-terminal domain. Jmjd2a is Fe+2 and α-ketoglutarate dependent. Methylation of histones in the chromatin is involved in regulation of gene activity, chromatin structure and epigenetic memory<ref>PMID:16677698</ref>.<br /> | + | *'''Jumonji domain-containing protein 2a''' (Jmjd2a) or '''KDM4A''' or '''Lysine-specific demethylase''' catalyzes the demethylation of trimethylated histone H3 at lysine residues 9 and 36 producing the dimethylated form. Jmjd2a domains contain: N-terminal, catalytic core (residues 1-350), 2 TUDOR domains which recognize dimethylated Arg (residues 895-1011) , 2 PHD-type zinc fingers and C-terminal domain. Jmjd2a is Fe+2 and α-ketoglutarate dependent. Methylation of histones in the chromatin is involved in regulation of gene activity, chromatin structure and epigenetic memory<ref>PMID:16677698</ref>.<br /> |
*'''Jumonji domain-containing protein 2b''' (Jmjd2b) or '''KDM4B''' and '''Jumonji domain-containing protein 2d''' (Jmjd2d) or '''KDM4D''' catalyze the demethylation of trimethylated histone H3 at lysine residues 9 producing the dimethylated form. <br /> | *'''Jumonji domain-containing protein 2b''' (Jmjd2b) or '''KDM4B''' and '''Jumonji domain-containing protein 2d''' (Jmjd2d) or '''KDM4D''' catalyze the demethylation of trimethylated histone H3 at lysine residues 9 producing the dimethylated form. <br /> | ||
*'''Jumonji domain-containing protein 3''' (Jmjd3) or '''KDM6B''' and '''Jumonji domain-containing protein 2d''' (Jmjd2d) or '''KDM4D''' catalyze the demethylation of trimethylated histone H3 at lysine residues 27 producing the dimethylated form<ref>PMID:22308433</ref>. <br /> | *'''Jumonji domain-containing protein 3''' (Jmjd3) or '''KDM6B''' and '''Jumonji domain-containing protein 2d''' (Jmjd2d) or '''KDM4D''' catalyze the demethylation of trimethylated histone H3 at lysine residues 27 producing the dimethylated form<ref>PMID:22308433</ref>. <br /> | ||
| Line 70: | Line 70: | ||
**[[4qxc]] – mJmj1a residues 36-364 + Ni + oxalylglycine + H3 peptide <br /> | **[[4qxc]] – mJmj1a residues 36-364 + Ni + oxalylglycine + H3 peptide <br /> | ||
**[[4qx8]], [[4qx7]], [[4qwn]] – mJmj1a residues 36-364 + Ni + oxoglutarate + H3 peptide <br /> | **[[4qx8]], [[4qx7]], [[4qwn]] – mJmj1a residues 36-364 + Ni + oxoglutarate + H3 peptide <br /> | ||
| + | |||
| + | * Lysine-specific demethylase 5C; KDM5C; JARID1C | ||
| + | |||
| + | **[[5fwj]] – hLSD5C Jmj domain + inhibitor<br /> | ||
| + | **[[2jrz]] – hLSD5C Bright/ARID domain - NMR<br /> | ||
| + | |||
| + | *lysine-specific demethylase 5D or JARID1D | ||
| + | |||
| + | **[[2yqe]] – hLSD5D ARID domain - NMR<br /> | ||
*Jmjd1b (Lysine-specific demethylase 2B; KDM2B) | *Jmjd1b (Lysine-specific demethylase 2B; KDM2B) | ||
Revision as of 08:16, 5 July 2017
| |||||||||||
3D structures of jumonji domain-containing protein
Updated on 05-July-2017
5pkb, 5pka, 5pk9, 5pk8, 5pk7, 5pk6, 5pk5, 5pk4, 5pk3, 5pk2, 5pk1, 5pk0,
5pjz, 5pjy, 5pjx, 5pjw, 5pjv, 5pju, 5pjt, 5pjs, 5pjr, 5pjq, 5pjp, 5pjo, 5pjn, 5pjm, 5pjl, 5pjk, 5pjj, 5pji, 5pjh, 5pjg, 5pjf, 5pje, 5pjd, 5pjc, 5pjb, 5pja, 5pj9, 5pj8, 5pj7, 5pj6, 5pj5, 5pj4, 5pj3, 5pj2, 5pj1, 5pj0, 5piz, 5piy, 5pix, 5piw, 5piv, 5piu, 5pit, 5pis, 5pir, 5piq, 5pip, 5pio, 5pin, 5pim, 5pil, 5pik, 5pij, 5pii, 5pih, 5pig, 5pif, 5pie, 5pid, 5pic, 5pib, 5pia, 5pi9, 5pi8, 5pi7, 5pi6, 5pi5, 5pi4, 5pi3, 5pi2, 5pi1, 5pi0, 5phz, 5phy, 5phx, 5phw, 5phv, 5phu, 5pht, 5phs, 5phr, 5phq, 5php, 5pho, 5phn, 5phm, 5phl, 5phk, 5phj, 5phi, 5phh, 5phg, 5phf, 5phe, 5phd, 5phc, 5phb, 5pha, 5ph9, 5ph8, 5ph7, 5ph6, 5ph5, 5ph4, 5ph3, 5ph2, 5ph1, 5ph0,
5pka, 5pk9, 5pk8, 5pk7, 5pk6, 5pk5, 5pk4, 5pk3, 5pk2, 5pk1, 5pk0, 5plj, 5pli, 5plh, 5plg – hJmjd2d catalytic domain + Zn
References
- ↑ Chen Z, Zang J, Whetstine J, Hong X, Davrazou F, Kutateladze TG, Simpson M, Mao Q, Pan CH, Dai S, Hagman J, Hansen K, Shi Y, Zhang G. Structural insights into histone demethylation by JMJD2 family members. Cell. 2006 May 19;125(4):691-702. Epub 2006 May 4. PMID:16677698 doi:http://dx.doi.org/10.1016/j.cell.2006.04.024
- ↑ Canovas S, Cibelli JB, Ross PJ. Jumonji domain-containing protein 3 regulates histone 3 lysine 27 methylation during bovine preimplantation development. Proc Natl Acad Sci U S A. 2012 Feb 14;109(7):2400-5. doi:, 10.1073/pnas.1119112109. Epub 2012 Jan 30. PMID:22308433 doi:http://dx.doi.org/10.1073/pnas.1119112109
- ↑ Lawrence P, Rai D, Conderino JS, Uddowla S, Rieder E. Role of Jumonji C-domain containing protein 6 (JMJD6) in infectivity of foot-and-mouth disease virus. Virology. 2016 Feb 18;492:38-52. doi: 10.1016/j.virol.2016.02.005. PMID:26896934 doi:http://dx.doi.org/10.1016/j.virol.2016.02.005
- ↑ Chen Z, Zang J, Kappler J, Hong X, Crawford F, Wang Q, Lan F, Jiang C, Whetstine J, Dai S, Hansen K, Shi Y, Zhang G. Structural basis of the recognition of a methylated histone tail by JMJD2A. Proc Natl Acad Sci U S A. 2007 Jun 26;104(26):10818-23. Epub 2007 Jun 13. PMID:17567753
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Joel L. Sussman, Jaime Prilusky