Sandbox Reserved 1053
From Proteopedia
| Line 3: | Line 3: | ||
== Background == | == Background == | ||
===Operon Overview=== | ===Operon Overview=== | ||
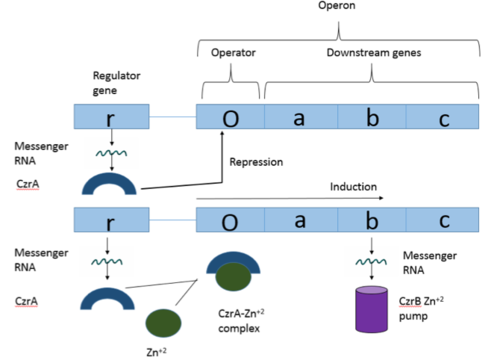
| - | [https://en.wikipedia.org/wiki/Operon Operons] are a critical genetic component of most prokaryotic cells. There are many different operons | + | [https://en.wikipedia.org/wiki/Operon Operons] are a critical genetic component of most prokaryotic cells. There are many different operons, responsible for the production of proteins with a wide range of functions. The most well-known and studied operons are the Lac and Trp operons, responsible for producing enzymes which metabolize lactose and tryptophan respectively. Despite many differences in each operon and the proteins that they encode, operons all function in the same general manner. Structurally, each operon contains a regulator, an operator, and one or more structural genes. The regulator gene codes for a protein responsible for managing the expression level of the structural genes. The operator contains the binding sequence for [https://en.wikipedia.org/wiki/RNA_polymerase RNA polymerase] and is the site where transcription begins. Lastly, the structural genes code for proteins to be used elsewhere. The regulator protein (produced as a result of expression of the regulator gene) most often acts in a repressive manner, though this is not always the case. That is, the regulator protein will bind to the operator, inhibiting the binding and/or progression of RNA polymerase to the structural genes, thus inhibiting transcription of the genes into mRNA. If the regulator protein were always active, there could never be adequate expression of the structural genes, so there must be a way to inactive the regulator protein, thus enabling expression of the structural genes. This is usually achieved through the binding of an inhibitor to the regulator protein. Since regulator proteins are DNA binding proteins, often this inhibition is allosteric rather than competitive, that is the inhibitor is not something that mimics DNA and binds to the active site physically blocking DNA from binding. Rather, the inhibitor of the regulator binds to somewhere other than the active site of the protein, changing it in some way which decreases the proteins affinity or ability to bind DNA and repress transcription. |
[[Image:Operon.png|500px|thumb|center|Figure 1:Overview of Operon Structure]] | [[Image:Operon.png|500px|thumb|center|Figure 1:Overview of Operon Structure]] | ||
| Line 10: | Line 10: | ||
===Czr Operon=== | ===Czr Operon=== | ||
| - | The Chromosome Determined Zinc Responsible (Czr) operon acts as described above, with Czr A acting as | + | The Chromosome Determined Zinc Responsible (Czr) operon acts as described above, with Czr A acting as a regulator protein. As such, Czr A is responsible for controlling the transcription of the rest of the operon and by extension, the transport of Zn <sup>2+</sup> out of the cell; the role of Czr A in the Czr operon is described in further detail as part of the explanation of biological function. In addition to being a component of an operon, Czr A is also considered to be a metal sensor protein. While the immediate function of Czr A is gene regulation, this serves the larger purpose of acting to maintain an appropriate concentration of Zn <sup>2+</sup> in the cell. |
| Line 16: | Line 16: | ||
Czr A is a transcriptional repressor protein responsible for the regulation of the Czr operon<ref name="critical">Arunkumar A., Campanello G., Giedroc D. (2009). Solution Structure of a | Czr A is a transcriptional repressor protein responsible for the regulation of the Czr operon<ref name="critical">Arunkumar A., Campanello G., Giedroc D. (2009). Solution Structure of a | ||
paradigm ArsR family zinc sensor in the DNA-bound state. PNAS 106:43 | paradigm ArsR family zinc sensor in the DNA-bound state. PNAS 106:43 | ||
| - | 18177-18182.</ref>. The Czr operon contains genes for the proteins Czr A and [http://proteopedia.org/wiki/index.php/3byr Czr B]. Czr B is a Zinc transport protein which moves Zn<sup>2+</sup> out of a cell while Czr A regulates this process by controlling expression level of Czr B. When relatively low amounts of zinc are present in the cell Czr A will bind to DNA, preventing the progression of RNA polymerase and thus inhibiting expression of Czr B. Decreased expression of Czr B results in the ability of the cell to retain Zn<sup>2+</sup> more readily. Because Czr A and Czr B are transcribed as part of the same operon, an inhibitor of Czr A must be readily available to allow full transcription of Czr B when necessary. Czr A is noncompetitively inhibited by the binding of two Zn<sup>2+</sup> ions, which is ideal in that this allows | + | 18177-18182.</ref>. The Czr operon contains genes for the proteins Czr A and [http://proteopedia.org/wiki/index.php/3byr Czr B]. Czr B is a Zinc transport protein which moves Zn<sup>2+</sup> out of a cell while Czr A regulates this process by controlling expression level of Czr B. When relatively low amounts of zinc are present in the cell Czr A will bind to DNA, preventing the progression of RNA polymerase and thus inhibiting expression of Czr B. Decreased expression of Czr B results in the ability of the cell to retain Zn<sup>2+</sup> more readily. Because Czr A and Czr B are transcribed as part of the same operon, an inhibitor of Czr A must be readily available to allow full transcription of Czr B when necessary. Czr A is noncompetitively inhibited by the binding of two Zn<sup>2+</sup> ions, which is ideal in that this allows expression of Czr B, a Zn<sup>2+</sup> transporter to be dependent on the relative amount of Zn<sup>2+</sup> in the cell. Czr A displays two different conformations; the first binds DNA and has relatively low affinity for Zn<sup>2+</sup> (PDB code: 2kjb). In this conformation the <scene name='69/694220/A5_helices__dna_binding/1'>a5 helices are aligned</scene>. Binding of zinc drives a conformational change (PDB code: 2kjc) in which the <scene name='69/694220/A5_helices_dna_binding/1'>a5 helices become unaligned</scene>, lowering the affinity for DNA. |
| - | + | ||
| - | + | ||
===Zinc Binding === | ===Zinc Binding === | ||
| - | Zinc acts as an inhibitor to Czr A<ref name="critical"/>, thus preventing transcriptional repression of Czr B and allowing Zn<sup>2+</sup> transport out of the cell. This allows for zinc transport to essentially be self regulated. That is, when zinc concentration in the cell is relatively high, zinc ions bind to Czr A, causing a conformational change which releases the bound DNA. DNA without Czr A bound is free to be transcribed and Czr B is again expressed, allowing for Zn<sup>2+</sup> transport out of the cell. | + | Zinc acts as an inhibitor to Czr A<ref name="critical"/>, thus preventing transcriptional repression of Czr B and allowing Zn<sup>2+</sup> transport out of the cell. This allows for zinc transport to essentially be self regulated. That is, when zinc concentration in the cell is relatively high, zinc ions bind to Czr A, causing a conformational change which releases the bound DNA. DNA without Czr A bound is free to be transcribed and Czr B is again expressed, allowing for Zn<sup>2+</sup> transport out of the cell. At low Zn<sup>2+</sup> concentrations, CzrA represses RNA Polymerase activity, and Zn<sup>2+</sup> ions are maintained inside the cell. |
== Structural Overview == | == Structural Overview == | ||
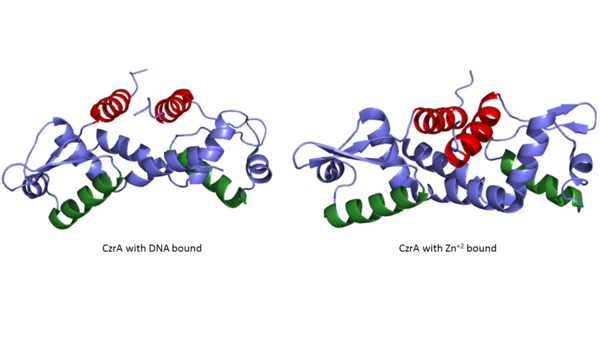
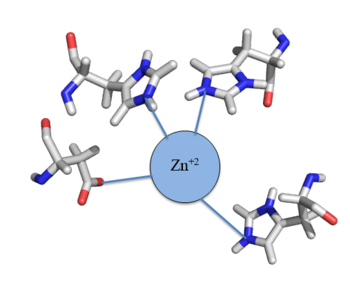
| - | CzrA functions as a [https://en.wikipedia.org/wiki/Dimer_(chemistry) dimer]. The <scene name='69/694218/Monomeric_unit/1'>monomeric units</scene> dimerize at the czr operon, repressing gene transcription. Each monomeric unit contains <scene name='69/694218/Helices/1'>five alpha helices</scene> seen in purple and <scene name='69/694218/B_sheets/1'>two beta sheets</scene> displayed in yellow. While the function of the [https://en.wikipedia.org/wiki/Beta_sheet beta sheets] are not yet known, key [https://en.wikipedia.org/wiki/Alpha_helix helices] regulate the binding of DNA and Zn<sup> +2 </sup>. The <scene name='69/694220/2kjb_colored_alpha_4/1'>alpha 4 helices</scene> are the location of DNA binding and the <scene name='69/694220/Zinc_pocket_with_residues/1'>alpha 5 helics</scene> contain the Zn<sup> +2 </sup> binding site. As Zn<sup> +2 </sup> binds | + | CzrA functions as a [https://en.wikipedia.org/wiki/Dimer_(chemistry) dimer]. The <scene name='69/694218/Monomeric_unit/1'>monomeric units</scene> dimerize at the czr operon, repressing gene transcription. Each monomeric unit contains <scene name='69/694218/Helices/1'>five alpha helices</scene> seen in purple and <scene name='69/694218/B_sheets/1'>two beta sheets</scene> displayed in yellow. While the function of the [https://en.wikipedia.org/wiki/Beta_sheet beta sheets] are not yet known, key [https://en.wikipedia.org/wiki/Alpha_helix helices] regulate the binding of DNA and Zn<sup>+2</sup>. The <scene name='69/694220/2kjb_colored_alpha_4/1'>alpha 4 helices</scene> are the location of DNA binding and the <scene name='69/694220/Zinc_pocket_with_residues/1'>alpha 5 helics</scene> contain the Zn<sup>+2</sup> binding site. As Zn<sup>+2</sup> ions binds the alpha 4 helices are <scene name='69/694220/2kjc_colored_alpha_4/1'>pushed out of alignment</scene>, repressing their DNA binding ability (Figure 2). Two seperate PDB codes exist for CzrA: CzrA with DNA bound (2KJB) and CzrA with zinc<sup>+2</sup> bound (2KJC). Unfortunately, zinc ions are not visible in the 2KJC NMR structure that was obtained for CzrA. |
[[Image:2KJB + 2KJC side by side.png|600px|center|thumb| Figure 2:Comparison of CzrA with Zn<sup>+2</sup> bound and CzrA with DNA bound with the alpha five helices shown in red and the alpha four helices shown in green]] | [[Image:2KJB + 2KJC side by side.png|600px|center|thumb| Figure 2:Comparison of CzrA with Zn<sup>+2</sup> bound and CzrA with DNA bound with the alpha five helices shown in red and the alpha four helices shown in green]] | ||
| - | == | + | == DNA Binding == |
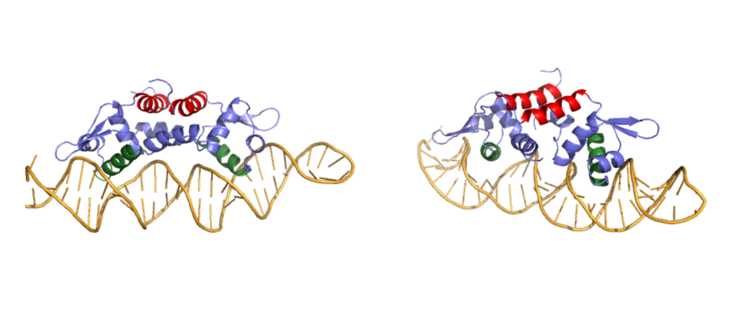
| - | + | Ser 54, Ser 57, and His 58 have been found to be the main <scene name='69/694219/Serandhisresidues/3'>sites of DNA interaction</scene>. <scene name='69/694220/2kjb_dna_alpha_4_helix/1'>These residues</scene> are likely to interact with the 5'-TGAA sequence found in the half-site of the DNA, where the alpha 4 helices <scene name='69/694219/Czra_with_dna/2'>form an interaction with DNA</scene>. These residues are found in the N terminal of the alpha 4 helix (figure 3). Additionally, Val 42 and Gln 53 are involved in the <scene name='69/694219/Dna_binding_pocket/1'>DNA binding pocket</scene>. This conclusion was experimentally determined by mutagenesis of the Gln and Val with Ala residues and measuring the mutant DNA binding capacity. In a previously published article <ref name="critical"/>, the DNA bound state of CzrA was tested by using the known critical residues for DNA interactions. <scene name='69/694220/Dna_binding_experiment/1'>Critical DNA binding residues</scene>, Gln53, Val42 (both shown in red), Ser54, Ser57, and His58 (all shown in orange), were replaced with Ala and then compared to the kinetics of the wild type protein. Replacing only the Q53 and V42 residues resulted in an 11-fold and 160-fold decrease in K<sub>a</sub>, respectively. Other residues such as S54, S57, and H58 were also replaced with Ala residues, and it was found that these mutations caused binding similar to the <scene name='69/694220/Dna_residues_when_inhibited/1'>fully inhibited Zn<sup>2+</sup> bound state</scene>. The conformational change that occurs from the Zinc to DNA bound state regarding these residues is small, but the alpha 4 helix (shown in green in Figure 2) does subtly move. Because no major physical change occurs between these two states, it further supports that this region is the main DNA interaction site because of the loss of affinity after the mutation took place. Table 1 in this same article shows the different K<sub>observed</sub>, and the measured decrease in K<sub>observed</sub> for each mutation. The bind between the DNA and the protein can be attributed to losing certain intermolecular forces such as possible hydrogen bonding when changing from Gln and Ala, and a loss of London Dispersion forces in the Val to Ala change. | |
The differences in binding favorability can also be seen when comparing the ΔG for the Apo-state vs. the DNA bound state and the Zinc vs. the Zinc and DNA bound state. These ΔGs were found to be -15.2kcal/mol and -9kcal/mol respectively<ref>DOI: 10.1021/ja208047b</ref>. This agrees with previously published data showing the Zinc binding inhibits the affinity the protein has to DNA. | The differences in binding favorability can also be seen when comparing the ΔG for the Apo-state vs. the DNA bound state and the Zinc vs. the Zinc and DNA bound state. These ΔGs were found to be -15.2kcal/mol and -9kcal/mol respectively<ref>DOI: 10.1021/ja208047b</ref>. This agrees with previously published data showing the Zinc binding inhibits the affinity the protein has to DNA. | ||
Revision as of 16:47, 9 August 2017
CzrA: A Zinc Dependent Transcriptional Regulator
|
Background
Operon Overview
Operons are a critical genetic component of most prokaryotic cells. There are many different operons, responsible for the production of proteins with a wide range of functions. The most well-known and studied operons are the Lac and Trp operons, responsible for producing enzymes which metabolize lactose and tryptophan respectively. Despite many differences in each operon and the proteins that they encode, operons all function in the same general manner. Structurally, each operon contains a regulator, an operator, and one or more structural genes. The regulator gene codes for a protein responsible for managing the expression level of the structural genes. The operator contains the binding sequence for RNA polymerase and is the site where transcription begins. Lastly, the structural genes code for proteins to be used elsewhere. The regulator protein (produced as a result of expression of the regulator gene) most often acts in a repressive manner, though this is not always the case. That is, the regulator protein will bind to the operator, inhibiting the binding and/or progression of RNA polymerase to the structural genes, thus inhibiting transcription of the genes into mRNA. If the regulator protein were always active, there could never be adequate expression of the structural genes, so there must be a way to inactive the regulator protein, thus enabling expression of the structural genes. This is usually achieved through the binding of an inhibitor to the regulator protein. Since regulator proteins are DNA binding proteins, often this inhibition is allosteric rather than competitive, that is the inhibitor is not something that mimics DNA and binds to the active site physically blocking DNA from binding. Rather, the inhibitor of the regulator binds to somewhere other than the active site of the protein, changing it in some way which decreases the proteins affinity or ability to bind DNA and repress transcription.
Structure Testing Area
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Arunkumar A., Campanello G., Giedroc D. (2009). Solution Structure of a paradigm ArsR family zinc sensor in the DNA-bound state. PNAS 106:43 18177-18182.
- ↑ Chakravorty DK, Wang B, Lee CW, Giedroc DP, Merz KM Jr. Simulations of allosteric motions in the zinc sensor CzrA. J Am Chem Soc. 2012 Feb 22;134(7):3367-76. doi: 10.1021/ja208047b. Epub 2011 Nov , 14. PMID:22007899 doi:http://dx.doi.org/10.1021/ja208047b
- ↑ MacPherson S, Larochelle M, Turcotte B. A fungal family of transcriptional regulators: the zinc cluster proteins. Microbiol Mol Biol Rev. 2006 Sep;70(3):583-604. PMID:16959962 doi:http://dx.doi.org/10.1128/MMBR.00015-06
- ↑ Miller J, McLachlan AD, Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun 4;4(6):1609-1614.
- ↑ Grossoehme NE, Giedroc DP. Energetics of allosteric negative coupling in the zinc sensor S. aureus CzrA. J Am Chem Soc. 2009 Dec 16;131(49):17860-70. doi: 10.1021/ja906131b. PMID:19995076 doi:http://dx.doi.org/10.1021/ja906131b