Sandbox Reserved 1053
From Proteopedia
| Line 26: | Line 26: | ||
== DNA Binding == | == DNA Binding == | ||
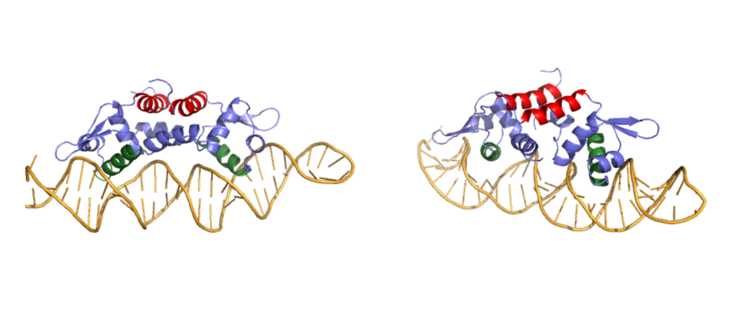
| - | Ser 54, Ser 57, and His 58 have been found to be the main <scene name='69/694219/Serandhisresidues/3'>sites of DNA interaction</scene>. <scene name='69/694220/2kjb_dna_alpha_4_helix/1'>These residues</scene> are likely to interact with the 5'-TGAA sequence found in the half-site of the DNA, where the alpha 4 helices <scene name='69/694219/Czra_with_dna/2'>form an interaction with DNA</scene>. These residues are found in the N terminal of the alpha 4 helix (figure 3). Additionally, Val 42 and Gln 53 are involved in the <scene name='69/694219/Dna_binding_pocket/1'>DNA binding pocket</scene>. This conclusion was experimentally determined by mutagenesis of the Gln and Val with Ala residues and measuring the mutant DNA binding capacity. In a previously published article <ref name="critical"/>, the DNA bound state of Czr A was tested by using the known critical residues for DNA interactions. <scene name='69/694220/Dna_binding_experiment/1'> Critical DNA binding residues</scene> Gln 53, Val 42 (red), Ser 54, Ser 57, and His 58 (orange) were individually mutated to Ala, and kinetic experiments were performed. Compared to wild type Czr A, mutating Gln53 and V42 residues resulted in an 11-fold and 160-fold decrease in K<sub>a</sub>, respectively. Mutations to the main DNA interaction sites Ser 54, Ser 57, and His 58 resulted in binding similar to the <scene name='69/694220/Dna_residues_when_inhibited/1'>fully inhibited Zn<sup>2+</sup> bound state</scene>. | + | Ser 54, Ser 57, and His 58 have been found to be the main <scene name='69/694219/Serandhisresidues/3'>sites of DNA interaction</scene>. <scene name='69/694220/2kjb_dna_alpha_4_helix/1'>These residues</scene> are likely to interact with the 5'-TGAA sequence found in the half-site of the DNA, where the alpha 4 helices <scene name='69/694219/Czra_with_dna/2'>form an interaction with DNA</scene>. These residues are found in the N terminal of the alpha 4 helix (figure 3). Additionally, Val 42 and Gln 53 are involved in the <scene name='69/694219/Dna_binding_pocket/1'>DNA binding pocket</scene>. This conclusion was experimentally determined by mutagenesis of the Gln and Val with Ala residues and measuring the mutant DNA binding capacity. In a previously published article <ref name="critical"/>, the DNA bound state of Czr A was tested by using the known critical residues for DNA interactions. <scene name='69/694220/Dna_binding_experiment/1'> Critical DNA binding residues</scene> Gln 53, Val 42 (red), Ser 54, Ser 57, and His 58 (orange) were individually mutated to Ala, and kinetic experiments were performed. Compared to wild type Czr A, mutating Gln53 and V42 residues resulted in an 11-fold and 160-fold decrease in K<sub>a</sub>, respectively. Mutations to the main DNA interaction sites Ser 54, Ser 57, and His 58 resulted in binding similar to the <scene name='69/694220/Dna_residues_when_inhibited/1'>fully inhibited Zn<sup>2+</sup> bound state</scene>, suggesting that these residues are essential to binding DNA. While the conformational change that occurs from the Zinc to DNA bound state of Czr A is small,the alpha 4 helices (shown in green in Figure 2) are slightly shifted. The loss of DNA binding in the mutagenesis experiements in combination with the lack of any other major physical changes between these two states further supports that the alpha 4 helices are the location of DNA binding in Czr A. Experimental data can be found in table 1 from this same article. |
| - | + | ||
| - | + | ||
[[Image:DNABound Final.PNG|750px|thumb|center| Figure 3:Side by side rotation showing the main DNA groove in CzrA]] | [[Image:DNABound Final.PNG|750px|thumb|center| Figure 3:Side by side rotation showing the main DNA groove in CzrA]] | ||
| Line 34: | Line 32: | ||
== Zinc Binding == | == Zinc Binding == | ||
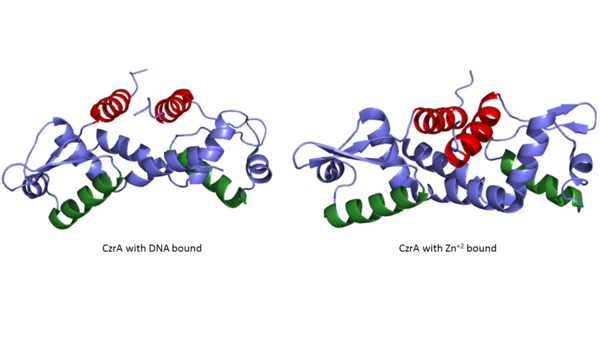
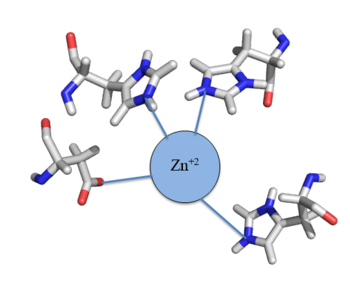
| - | Many zinc-dependent proteins are transcriptional regulators<ref>DOI: 10.1128/MMBR.00015-06</ref>. | + | Many zinc-dependent proteins are transcriptional regulators<ref>DOI: 10.1128/MMBR.00015-06</ref>. Czr A fits into this category as an [https://en.wikipedia.org/wiki/Allosteric_regulation allosteric inhibitor] of the czr operon. Two [https://en.wikipedia.org/wiki/Zinc Zn<sup> +2</sup>] ions may bind to the dimer<ref name="critical"/>, at the location of the <scene name='69/694218/Alpha_5_helices/2'> alpha 5 </scene> helix from each monomer. As zinc binds, the alpha 5 helices <scene name='69/694218/2kjc_zinc_bound/1'>unalign</scene> to inhibit the DNA binding residues (Figure 2). Furthermore, CzrA must be in its dimer form for zinc to bind. The <scene name='69/694218/Spacefill_with_zinc_pockets/1'>zinc binding pocket</scene> is formed by two residues from each monomer, so Zn<sup>+2</sup> cannot bind to the monomer. The <scene name='69/694220/Zinc_binding_residues/6'>zinc binding site</scene> is formed by Asp 84 and His 86 from one monomer, and His 97 and His 100 from the other monomer. Zinc ions were not present in the solution NMR crystal structure, so a representation of a zinc ion in the binding pocket can be seen in figure 4. Histidine residues are a repetitive and commonly found residue in zinc-binding proteins <ref>Miller J, McLachlan AD, Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun 4;4(6):1609-1614.</ref>. |
[[Image:Zinc tetrahedral complex.PNG|350px|thumb|center| Figure 4:Zn<sup>+2</sup> tetrahedral binding complex]] | [[Image:Zinc tetrahedral complex.PNG|350px|thumb|center| Figure 4:Zn<sup>+2</sup> tetrahedral binding complex]] | ||
Revision as of 17:53, 9 August 2017
CzrA: A Zinc Dependent Transcriptional Regulator
|
Background
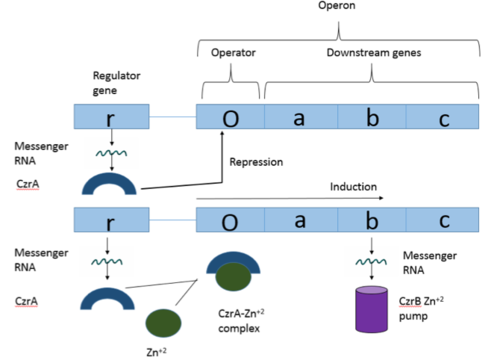
Operon Overview
Operons are a critical genetic component of most prokaryotic cells. There are many different operons, responsible for the production of proteins with a wide range of functions. The most well-known and studied operons are the Lac and Trp operons, responsible for producing enzymes which metabolize lactose and tryptophan respectively. Despite many differences in each operon and the proteins that they encode, operons all function in the same general manner. Structurally, each operon contains a regulator, an operator, and one or more structural genes. The regulator gene codes for a protein responsible for managing the expression level of the structural genes. The operator contains the binding sequence for RNA polymerase and is the site where transcription begins. Lastly, the structural genes code for proteins to be used elsewhere. The regulator protein (produced as a result of expression of the regulator gene) most often acts in a repressive manner, though this is not always the case. That is, the regulator protein will bind to the operator, inhibiting the binding and/or progression of RNA polymerase to the structural genes, thus inhibiting transcription of the genes into mRNA. If the regulator protein were always active, there could never be adequate expression of the structural genes, so there must be a way to inactive the regulator protein, thus enabling expression of the structural genes. This is usually achieved through the binding of an inhibitor to the regulator protein. Since regulator proteins are DNA binding proteins, often this inhibition is allosteric rather than competitive, that is the inhibitor is not something that mimics DNA and binds to the active site physically blocking DNA from binding. Rather, the inhibitor of the regulator binds to somewhere other than the active site of the protein, changing it in some way which decreases the proteins affinity or ability to bind DNA and repress transcription.
Structure Testing Area
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Arunkumar A., Campanello G., Giedroc D. (2009). Solution Structure of a paradigm ArsR family zinc sensor in the DNA-bound state. PNAS 106:43 18177-18182.
- ↑ MacPherson S, Larochelle M, Turcotte B. A fungal family of transcriptional regulators: the zinc cluster proteins. Microbiol Mol Biol Rev. 2006 Sep;70(3):583-604. PMID:16959962 doi:http://dx.doi.org/10.1128/MMBR.00015-06
- ↑ Miller J, McLachlan AD, Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun 4;4(6):1609-1614.
- ↑ Grossoehme NE, Giedroc DP. Energetics of allosteric negative coupling in the zinc sensor S. aureus CzrA. J Am Chem Soc. 2009 Dec 16;131(49):17860-70. doi: 10.1021/ja906131b. PMID:19995076 doi:http://dx.doi.org/10.1021/ja906131b