We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
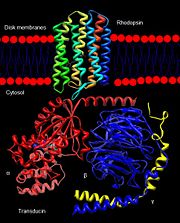
Rhodopsin
From Proteopedia
(Difference between revisions)
| Line 82: | Line 82: | ||
**[[5awz]], [[5ax0]], [[5ax1]] – AaRn-1 + retinal<br /> | **[[5awz]], [[5ax0]], [[5ax1]] – AaRn-1 + retinal<br /> | ||
**[[4tl3]] – AaRn-1 (mutant) <br /> | **[[4tl3]] – AaRn-1 (mutant) <br /> | ||
| - | **[[2x72]] – bRn + retinal + guanine nucleotide-binding protein peptide | + | **[[5te5]], [[5te3]] – bRn - bovine<br /> |
| + | **[[2x72]] – bRn + retinal + guanine nucleotide-binding protein peptide <br /> | ||
**[[3qc9]] – bRn + Mg + ADP<br /> | **[[3qc9]] – bRn + Mg + ADP<br /> | ||
**[[3oax]], [[3c9l]], [[2ped]], [[2i35]], [[2i36]], [[2i37]], [[1u19]], [[1gzm]], [[1l9h]], [[1hzx]] - bRn + retinal<br /> | **[[3oax]], [[3c9l]], [[2ped]], [[2i35]], [[2i36]], [[2i37]], [[1u19]], [[1gzm]], [[1l9h]], [[1hzx]] - bRn + retinal<br /> | ||
| + | **[[6fk6]], [[6fk7]], [[6fk8]], [[6fk9]], [[6fka]], [[6fkb]], [[6fkc]], [[6fkd]], [[5dys]] - bRn (mutant) + detergents + retinal derivative<br /> | ||
**[[3cap]], [[5te3]] - bRn <br /> | **[[3cap]], [[5te3]] - bRn <br /> | ||
**[[1jfp]], [[1f88]], [[5te5]] - bRn + retinal<br /> | **[[1jfp]], [[1f88]], [[5te5]] - bRn + retinal<br /> | ||
| Line 97: | Line 99: | ||
**[[1fdf]] – bRn helix 7 - NMR<BR /> | **[[1fdf]] – bRn helix 7 - NMR<BR /> | ||
**[[4pxf]] – bRn + arrestin peptide<br /> | **[[4pxf]] – bRn + arrestin peptide<br /> | ||
| + | **[[5wkt]] – bRn + transducin peptide<br /> | ||
**[[1xio]] – AnRn - ''Anabaena''<br /> | **[[1xio]] – AnRn - ''Anabaena''<br /> | ||
**[[2m3g]] – AnRn – NMR<br /> | **[[2m3g]] – AnRn – NMR<br /> | ||
**[[4tl3]] - AnRn (mutant) + retinal<br /> | **[[4tl3]] - AnRn (mutant) + retinal<br /> | ||
**[[4hyj]] - Rn + retinal + lipid – ''Exiguobacterium Sibiricum''<br /> | **[[4hyj]] - Rn + retinal + lipid – ''Exiguobacterium Sibiricum''<br /> | ||
| + | **[[5ax1]], [[5ax0]], [[5awz]] - Rn + retinal + lipid – ''Acetabularia acetabulum''<br /> | ||
| + | |||
| + | *Sensory rhodopsin II | ||
| + | |||
| + | **[[1gu8]], [[1gue]], [[1h68]], [[1jgj]], [[3qap]], [[3qdc]] – NpSRII – ''Natronomonas pharaonis''<br /> | ||
| + | **[[2ksy]] – NpSRII - NMR<br /> | ||
| + | **[[1h2s]], [[2f93]], [[2f95]], [[5jjn]], [[5jjj]], [[5jjf]], [[5jje]] – NpSRII + Rn II transducer<br /> | ||
| + | **[[4gyc]] - NpSRII (mutant) + Rn II transducer<br /> | ||
*Sodium pump rhodopsin | *Sodium pump rhodopsin | ||
| - | **[[4xtl]], [[4xtn]], [[4xto]], [[3x3b]], [[3x3c]], [[5jrf]] – | + | **[[4xtl]], [[4xtn]], [[4xto]], [[3x3b]], [[3x3c]], [[5jrf]] – DeRn – ''Dokdonia eikastra''<br /> |
| + | **[[5jrf]] – DeRn + detergent + I<br /> | ||
*Chloride pump rhodopsin | *Chloride pump rhodopsin | ||
| Line 124: | Line 136: | ||
**[[2hpy]] - bLRn + retinal<br /> | **[[2hpy]] - bLRn + retinal<br /> | ||
| + | |||
| + | *Halorhodopsin | ||
| + | |||
| + | **[[1e12]], [[2jaf]], [[5ahy]], [[5ahz]] - HRn + detergents + retinal – ''Halobacterium salinarium''<br /> | ||
| + | **[[2jag]] - HRn (mutant) + detergents + retinal<br /> | ||
| + | **[[3a7k]], [[3abw]], [[3qbg]], [[3qbi]], [[3qbk]], [[3qbl]], [[3vvk]] – NpRn + detergents + retinal<br /> | ||
| + | |||
| + | *Archaerhodopsin | ||
| + | |||
| + | **[[1uaz]] – HRn-1 + retinal – ''Halobacterium'' <br /> | ||
| + | **[[1vgo]], [[2z55, [[3wqj]] - HRn-2 + retinal<br /> | ||
| + | **[[2ei4]] - HRn-2 + retinal + bacterioruberin<br /> | ||
| + | |||
| + | *Proteorhodopsin | ||
| + | |||
| + | **[[2l6x]] - GpPRn + detergents + retinal – ''Gamma proteobacterium''<br /> | ||
| + | **[[4kly]], [[4knf]] - GpPRn + retinal<br /> | ||
| + | **[[4jq6]] – PRn + retinal + lipid<br /> | ||
| + | |||
| + | *Xanthorhodopsin | ||
| + | |||
| + | **[[3ddl]] - XRn + detergents + retinal – ''Salinibacter ruber''<br /> | ||
| + | |||
| + | *Deltarhodopsin | ||
| + | |||
| + | **[[4fbz]] - Rn + bacterioruberin + retinal – ''Haloterrigena thermotolerans''<br /> | ||
| + | |||
Revision as of 10:41, 6 September 2018
| |||||||||||
3D structures of rhodopsin
Updated on 06-September-2018 {{#tree:id=OrganizedByTopic|openlevels=0|
- Rhodopsin (Rn)
- 3aym, 3ayn, 2z73, 2ziy, 4ww3 – Rn + retinal – Flying squid
- 3am6 – AaRn-2 + cholesterol + retinal – Acetabularia acetabulum
- 5awz, 5ax0, 5ax1 – AaRn-1 + retinal
- 4tl3 – AaRn-1 (mutant)
- 5te5, 5te3 – bRn - bovine
- 2x72 – bRn + retinal + guanine nucleotide-binding protein peptide
- 3qc9 – bRn + Mg + ADP
- 3oax, 3c9l, 2ped, 2i35, 2i36, 2i37, 1u19, 1gzm, 1l9h, 1hzx - bRn + retinal
- 6fk6, 6fk7, 6fk8, 6fk9, 6fka, 6fkb, 6fkc, 6fkd, 5dys - bRn (mutant) + detergents + retinal derivative
- 3cap, 5te3 - bRn
- 1jfp, 1f88, 5te5 - bRn + retinal
- 3c9m, 5dys - bRn (mutant) + retinal
- 2j4y, 4bez - bRn (mutant)
- 3dqb, 4a4m, 4j4q - bRn + guanine nucleotide-binding protein peptide
- 4bey - bRn (mutant) + guanine nucleotide-binding protein peptide
- 5en0 - bRN (mutant) + guanine nucleotide-binding protein peptide + retinal
- 1vqx, 1nzs – bRn C terminal – NMR
- 1edx - bRn N terminal – NMR
- 1eds, 1edv, 1edw – bRn intradiskal loop – NMR
- 1fdf – bRn helix 7 - NMR
- 4pxf – bRn + arrestin peptide
- 5wkt – bRn + transducin peptide
- 1xio – AnRn - Anabaena
- 2m3g – AnRn – NMR
- 4tl3 - AnRn (mutant) + retinal
- 4hyj - Rn + retinal + lipid – Exiguobacterium Sibiricum
- 5ax1, 5ax0, 5awz - Rn + retinal + lipid – Acetabularia acetabulum
- 3aym, 3ayn, 2z73, 2ziy, 4ww3 – Rn + retinal – Flying squid
- Sensory rhodopsin II
- Sodium pump rhodopsin
- Chloride pump rhodopsin
- Metarhodopsin II
- Bathorhodopsin
- 2g87 - bBRn + retinal
- 2g87 - bBRn + retinal
- Lumirhodopsin
- 2hpy - bLRn + retinal
- 2hpy - bLRn + retinal
- Halorhodopsin
- Archaerhodopsin
- Proteorhodopsin
- Xanthorhodopsin
- 3ddl - XRn + detergents + retinal – Salinibacter ruber
- 3ddl - XRn + detergents + retinal – Salinibacter ruber
- Deltarhodopsin
- 4fbz - Rn + bacterioruberin + retinal – Haloterrigena thermotolerans
- 4fbz - Rn + bacterioruberin + retinal – Haloterrigena thermotolerans
}}
References
- ↑ Hornak V, Ahuja S, Eilers M, Goncalves JA, Sheves M, Reeves PJ, Smith SO. Light activation of rhodopsin: insights from molecular dynamics simulations guided by solid-state NMR distance restraints. J Mol Biol. 2010 Feb 26;396(3):510-27. Epub 2009 Dec 11. PMID:20004206 doi:10.1016/j.jmb.2009.12.003
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Sakmar TP. Structure of rhodopsin and the superfamily of seven-helical receptors: the same and not the same. Curr Opin Cell Biol. 2002 Apr;14(2):189-95. PMID:11891118
- ↑ 3.0 3.1 3.2 3.3 Kristiansen K. Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol Ther. 2004 Jul;103(1):21-80. PMID:15251227 doi:10.1016/j.pharmthera.2004.05.002
- ↑ Millar RP, Newton CL. The year in G protein-coupled receptor research. Mol Endocrinol. 2010 Jan;24(1):261-74. Epub 2009 Dec 17. PMID:20019124 doi:10.1210/me.2009-0473
- ↑ 5.0 5.1 5.2 Meng EC, Bourne HR. Receptor activation: what does the rhodopsin structure tell us? Trends Pharmacol Sci. 2001 Nov;22(11):587-93. PMID:11698103
- ↑ 6.0 6.1 Shieh T, Han M, Sakmar TP, Smith SO. The steric trigger in rhodopsin activation. J Mol Biol. 1997 Jun 13;269(3):373-84. PMID:9199406 doi:10.1006/jmbi.1997.1035
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 Okada T, Ernst OP, Palczewski K, Hofmann KP. Activation of rhodopsin: new insights from structural and biochemical studies. Trends Biochem Sci. 2001 May;26(5):318-24. PMID:11343925
- ↑ 8.0 8.1 Okada T, Sugihara M, Bondar AN, Elstner M, Entel P, Buss V. The retinal conformation and its environment in rhodopsin in light of a new 2.2 A crystal structure. J Mol Biol. 2004 Sep 10;342(2):571-83. PMID:15327956 doi:10.1016/j.jmb.2004.07.044
- ↑ 9.0 9.1 Janz JM, Farrens DL. Assessing structural elements that influence Schiff base stability: mutants E113Q and D190N destabilize rhodopsin through different mechanisms. Vision Res. 2003 Dec;43(28):2991-3002. PMID:14611935
- ↑ 10.0 10.1 10.2 Kisselev OG. Focus on molecules: rhodopsin. Exp Eye Res. 2005 Oct;81(4):366-7. PMID:16051215 doi:10.1016/j.exer.2005.06.018
- ↑ 11.0 11.1 11.2 Verhoeven MA, Bovee-Geurts PH, de Groot HJ, Lugtenburg J, DeGrip WJ. Methyl substituents at the 11 or 12 position of retinal profoundly and differentially affect photochemistry and signalling activity of rhodopsin. J Mol Biol. 2006 Oct 13;363(1):98-113. Epub 2006 Jul 28. PMID:16962138 doi:10.1016/j.jmb.2006.07.039
- ↑ 12.0 12.1 12.2 12.3 Morris MB, Dastmalchi S, Church WB. Rhodopsin: structure, signal transduction and oligomerisation. Int J Biochem Cell Biol. 2009 Apr;41(4):721-4. Epub 2008 Aug 3. PMID:18692154 doi:10.1016/j.biocel.2008.04.025
- ↑ 13.0 13.1 13.2 13.3 13.4 Nelson, D., and Cox, M. Lehninger Principles of Biochemistry. 2008. 5th edition. W. H. Freeman and Company, New York, New York, USA. pp. 462-465.
- ↑ Hurley JB, Spencer M, Niemi GA. Rhodopsin phosphorylation and its role in photoreceptor function. Vision Res. 1998 May;38(10):1341-52. PMID:9667002
- ↑ 15.0 15.1 15.2 Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature. 2008 Jul 10;454(7201):183-7. Epub 2008 Jun 18. PMID:18563085 doi:10.1038/nature07063
- ↑ 16.0 16.1 Surya A, Knox BE. Enhancement of opsin activity by all-trans-retinal. Exp Eye Res. 1998 May;66(5):599-603. PMID:9628807 doi:10.1006/exer.1997.0453
See Also
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Wayne Decatur, Jaime Prilusky, Joel L. Sussman, Cinting Lim