We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Journal:Structure:1
From Proteopedia
(Difference between revisions)

| Line 12: | Line 12: | ||
The mutations severely destabilized the protein, making them accessible only on a pre-stabilized TEM1 variant. The X-ray structure of the complex formed between the mutant TEM1 (termed T1 G268A) and wild-type TEM1 showed the two proteins to be related by a pseudo-two-fold symmetry axis (<scene name='76/763767/Cv/10'>Figure 2</scene>). The interface is comprised of strand 2 from both proteins forming a <scene name='76/763767/Cv/11'>continuous β-sheet</scene>, which spans to strand 1 and forms a <scene name='76/763767/Cv/13'>perfect backbone hydrogen bond network</scene>. The most surprising feature of the complex is that helix 1 of T1 G268A is completely missing in the density map. <scene name='76/763767/Cv/14'>Overlaying TEM1-WT onto T1 G268A</scene> shows that a properly folded N'-helix in T1 G268A would physically interfere with the interaction, <scene name='76/763767/Cv/15'>making complex formation impossible</scene>. | The mutations severely destabilized the protein, making them accessible only on a pre-stabilized TEM1 variant. The X-ray structure of the complex formed between the mutant TEM1 (termed T1 G268A) and wild-type TEM1 showed the two proteins to be related by a pseudo-two-fold symmetry axis (<scene name='76/763767/Cv/10'>Figure 2</scene>). The interface is comprised of strand 2 from both proteins forming a <scene name='76/763767/Cv/11'>continuous β-sheet</scene>, which spans to strand 1 and forms a <scene name='76/763767/Cv/13'>perfect backbone hydrogen bond network</scene>. The most surprising feature of the complex is that helix 1 of T1 G268A is completely missing in the density map. <scene name='76/763767/Cv/14'>Overlaying TEM1-WT onto T1 G268A</scene> shows that a properly folded N'-helix in T1 G268A would physically interfere with the interaction, <scene name='76/763767/Cv/15'>making complex formation impossible</scene>. | ||
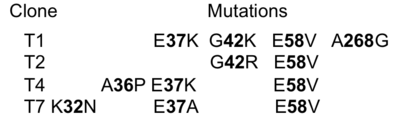
| - | A closer look at the three mutations evolved during selection shows that two of them (E37K and E58V) are important in stabilizing the N'-helix in its position. Analysis of the <scene name='76/763767/Cv/8'>WT structure</scene> ([[1btl]]) shows that <scene name='76/763767/Cv/5'>E37 forms an intra-protein salt bridge with R61</scene>, which in turn <scene name='76/763767/Cv/6'>interacts with E64</scene>, which <scene name='76/763767/Cv/7'>interacts with R43</scene>. <scene name='76/763767/Cv/9'>E58 interacts with the NH2-terminus of TEM1-WT</scene>. Therefore, loosing E37 and E58 will destabilize the N'- helix, as indeed seen in the T1 structure <scene name='76/763767/Cv/10'>Figure 2</scene>. The two additional selected mutations, K32 and A36 in some of the clones probably also contribute to destabilization of the N'helix. The only selected mutation located directly in the binding interface is E58V, which is present in all four selected clones. E58V forms hydrophobic contacts with L30 and F60 of the other chain. The structure implies that the formed interaction would be very specific between the evolved mutants and TEM1-WT. The structure of the complex shows that one copy of the N'-helix is important in stabilizing the interaction between the two proteins. Hence the WT cannot dimerize since the N'-helix physically interferes with the interaction while the selected TEM1 proteins are missing a folded N'-helix. A detailed map of the interactions formed between the two proteins is presented in Figure 4. | + | A closer look at the three mutations evolved during selection shows that two of them (E37K and E58V) are important in stabilizing the N'-helix in its position. Analysis of the <scene name='76/763767/Cv/8'>WT structure</scene> ([[1btl]]) shows that <scene name='76/763767/Cv/5'>E37 forms an intra-protein salt bridge with R61</scene>, which in turn <scene name='76/763767/Cv/6'>interacts with E64</scene>, which <scene name='76/763767/Cv/7'>interacts with R43</scene>. <scene name='76/763767/Cv/9'>E58 interacts with the NH2-terminus of TEM1-WT</scene>. Therefore, loosing E37 and E58 will destabilize the N'- helix, as indeed seen in the T1 structure (<scene name='76/763767/Cv/10'>Figure 2</scene>). The two additional selected mutations, K32 and A36 in some of the clones probably also contribute to destabilization of the N'helix. The only selected mutation located directly in the binding interface is E58V, which is present in all four selected clones. E58V forms hydrophobic contacts with L30 and F60 of the other chain. The structure implies that the formed interaction would be very specific between the evolved mutants and TEM1-WT. The structure of the complex shows that one copy of the N'-helix is important in stabilizing the interaction between the two proteins. Hence the WT cannot dimerize since the N'-helix physically interferes with the interaction while the selected TEM1 proteins are missing a folded N'-helix. A detailed map of the interactions formed between the two proteins is presented in Figure 4. |
</StructureSection> | </StructureSection> | ||
Revision as of 11:13, 22 October 2017
| |||||||||||
- ↑ REF
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.