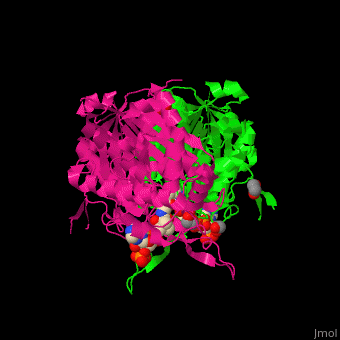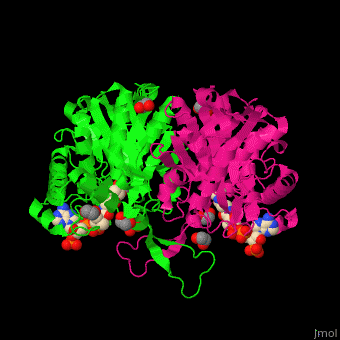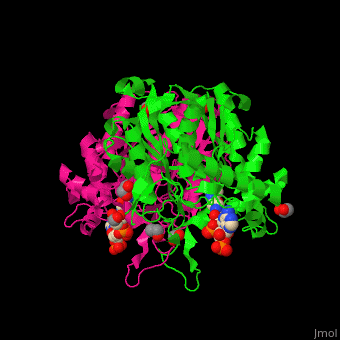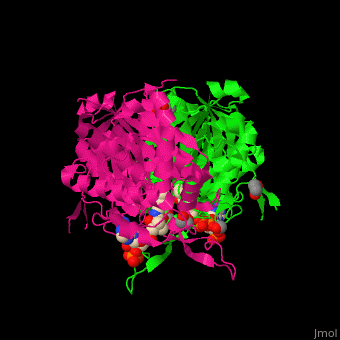We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Thiolase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <StructureSection load='4c2j' size=' | + | <StructureSection load='4c2j' size='350' side='right' caption='Human 3-ketoacyl-CoA complex with CoA and ethylene glycol(PDB code [[4c2j]]).' scene='44/447919/Cv/7'> |
== Function == | == Function == | ||
Two different classes of '''thiolases''' are found.<br /> | Two different classes of '''thiolases''' are found.<br /> | ||
| Line 39: | Line 39: | ||
**[[4ubv]] – MtKCT + CoA + acetyl CoA <br /> | **[[4ubv]] – MtKCT + CoA + acetyl CoA <br /> | ||
| - | *Acetoacetyl-CoA thiolase | + | *Acetoacetyl-CoA thiolase or acetyl-CoA acetyltransferase |
**[[1m4s]], [[1m4t]], [[1dlu]], [[1qfl]] - ZrACT – ''Zoogloea ramigera''<br /> | **[[1m4s]], [[1m4t]], [[1dlu]], [[1qfl]] - ZrACT – ''Zoogloea ramigera''<br /> | ||
| Line 50: | Line 50: | ||
**[[5f0v]], [[5f38]], [[4wys]] – ACT – ''Escherichia coli'' <br /> | **[[5f0v]], [[5f38]], [[4wys]] – ACT – ''Escherichia coli'' <br /> | ||
**[[4o9a]], [[4o99]], [[4o9c]], [[4nzs]] – ReACT <br /> | **[[4o9a]], [[4o99]], [[4o9c]], [[4nzs]] – ReACT <br /> | ||
| + | **[[5xyj]], [[5xz5]] – yACT <br /> | ||
*Acetoacetyl-CoA thiolase complex | *Acetoacetyl-CoA thiolase complex | ||
| Line 60: | Line 61: | ||
**[[1wl4]] – hACT cytosolic + CoA <br /> | **[[1wl4]] – hACT cytosolic + CoA <br /> | ||
**[[4xl4]] – CaACT + CoA <br /> | **[[4xl4]] – CaACT + CoA <br /> | ||
| - | + | **[[6et9]] – MtACT + PFAM DUF35 + hydroxymethylglutaryl-CoA synthase - ''Methanothermococcus thermolithotrophicus''<br /> | |
| + | **[[6esq]] – MtACT + CoA + PFAM DUF35 + hydroxymethylglutaryl-CoA synthase <br /> | ||
| + | **[[6are]] – ACT + CoA + acetate - ''Aspergillus fumigatus'' <br /> | ||
}} | }} | ||
== References == | == References == | ||
<references/> | <references/> | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Revision as of 19:17, 25 September 2018
| |||||||||||
3D Structures of Thiolase
Updated on 25-September-2018
References
- ↑ Sundaramoorthy R, Micossi E, Alphey MS, Germain V, Bryce JH, Smith SM, Leonard GA, Hunter WN. The crystal structure of a plant 3-ketoacyl-CoA thiolase reveals the potential for redox control of peroxisomal fatty acid beta-oxidation. J Mol Biol. 2006 Jun 2;359(2):347-57. Epub 2006 Mar 29. PMID:16630629 doi:http://dx.doi.org/10.1016/j.jmb.2006.03.032
- ↑ Soto G, Stritzler M, Lisi C, Alleva K, Pagano ME, Ardila F, Mozzicafreddo M, Cuccioloni M, Angeletti M, Ayub ND. Acetoacetyl-CoA thiolase regulates the mevalonate pathway during abiotic stress adaptation. J Exp Bot. 2011 Nov;62(15):5699-711. doi: 10.1093/jxb/err287. Epub 2011 Sep 9. PMID:21908473 doi:http://dx.doi.org/10.1093/jxb/err287
- ↑ Kiema TR, Harijan RK, Strozyk M, Fukao T, Alexson SE, Wierenga RK. The crystal structure of human mitochondrial 3-ketoacyl-CoA thiolase (T1): insight into the reaction mechanism of its thiolase and thioesterase activities. Acta Crystallogr D Biol Crystallogr. 2014 Dec 1;70(Pt 12):3212-25. doi:, 10.1107/S1399004714023827. Epub 2014 Nov 22. PMID:25478839 doi:http://dx.doi.org/10.1107/S1399004714023827