This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Chiara Evans/Sandbox 1
From Proteopedia
| Line 1: | Line 1: | ||
| - | + | ==ATAD2 Bromodomain== | |
| - | ATAD2 Bromodomain | + | |
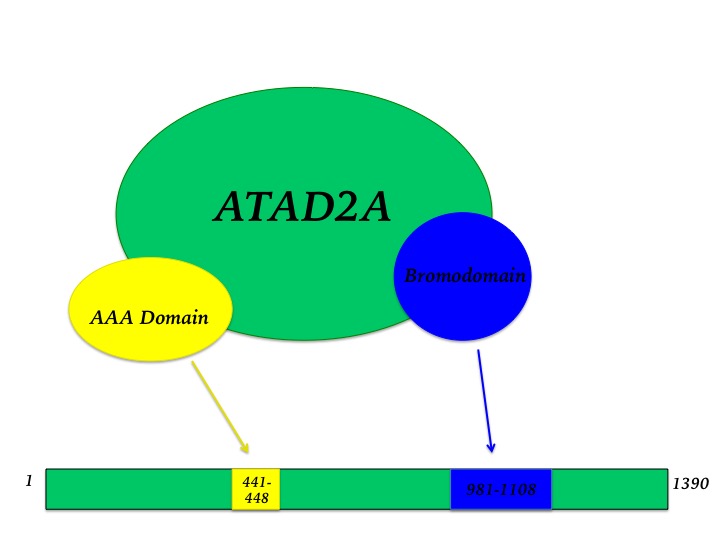
The ATPase family, AAA-domain containing protein 2 (ATAD2) is a nuclear co-regulator protein with an ATPase domain and a bromodomain, whose function remains largely undefined. AAA ATPase superfamily members are associated with varied cellular activities in which the energy from ATP is harnessed to produce a mechanical force for molecular remodeling. Bromodomains are known to bind acetyl-lysines and are thought to regulate protein interactions and in addition to gene transcription. Studies have shown ATAD2 (also called ANCCA) to play a role in estrogen and androgen receptor signaling and also act as a co-activator of the MYC oncogene. ATAD2 is found overexpressed in numerous types of cancer and is correlated with poor patient prognosis. This makes ATAD2 an attractive target for novel cancer therapeutics. A general idea of the ATAD2 protein is given in Figure 1. | The ATPase family, AAA-domain containing protein 2 (ATAD2) is a nuclear co-regulator protein with an ATPase domain and a bromodomain, whose function remains largely undefined. AAA ATPase superfamily members are associated with varied cellular activities in which the energy from ATP is harnessed to produce a mechanical force for molecular remodeling. Bromodomains are known to bind acetyl-lysines and are thought to regulate protein interactions and in addition to gene transcription. Studies have shown ATAD2 (also called ANCCA) to play a role in estrogen and androgen receptor signaling and also act as a co-activator of the MYC oncogene. ATAD2 is found overexpressed in numerous types of cancer and is correlated with poor patient prognosis. This makes ATAD2 an attractive target for novel cancer therapeutics. A general idea of the ATAD2 protein is given in Figure 1. | ||
Revision as of 21:38, 1 December 2017
Contents |
ATAD2 Bromodomain
The ATPase family, AAA-domain containing protein 2 (ATAD2) is a nuclear co-regulator protein with an ATPase domain and a bromodomain, whose function remains largely undefined. AAA ATPase superfamily members are associated with varied cellular activities in which the energy from ATP is harnessed to produce a mechanical force for molecular remodeling. Bromodomains are known to bind acetyl-lysines and are thought to regulate protein interactions and in addition to gene transcription. Studies have shown ATAD2 (also called ANCCA) to play a role in estrogen and androgen receptor signaling and also act as a co-activator of the MYC oncogene. ATAD2 is found overexpressed in numerous types of cancer and is correlated with poor patient prognosis. This makes ATAD2 an attractive target for novel cancer therapeutics. A general idea of the ATAD2 protein is given in Figure 1.
Structural Analysis
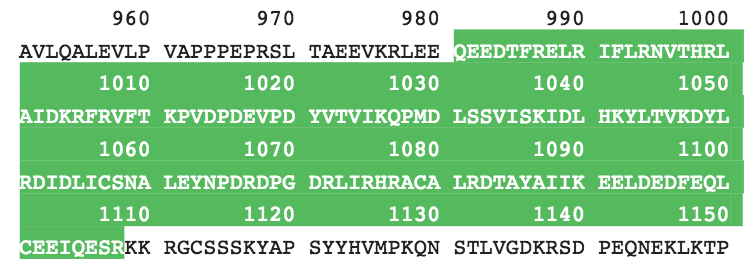
The ATAD2 bromodomain contains the canonical left-handed bundle of 4 alpha helices denoted aZ, aA, aB, and aC. These maintain two variable loops, the ZA loop and the BC loop. Variations in these loops confer specificity for different acetylated lysine modifications. The protein sequence is given in Figure 3. The bromodomain is contained within residues 981-1108 of the entire protein sequence of ATAD2. It is composed of 128 amino acid residues, and includes all of the twenty common amino acids but tryptophan. This gives a molecular weight of 15212.24 Daltons. The ATAD2 bromodomain belongs to the bromodomain superfamily. The apo structure of the ATAD2 bromodomain (PDBID: 3DAI) was solved with X-ray crystallography by Filipakopoulos et al. in 2012 and reveals the tertiary structure as described above and seen on this page.
Relation to other Family IV Bromodomains
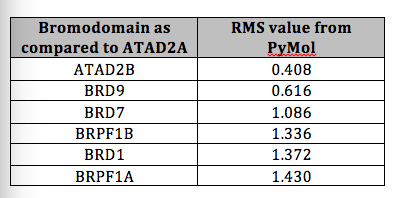
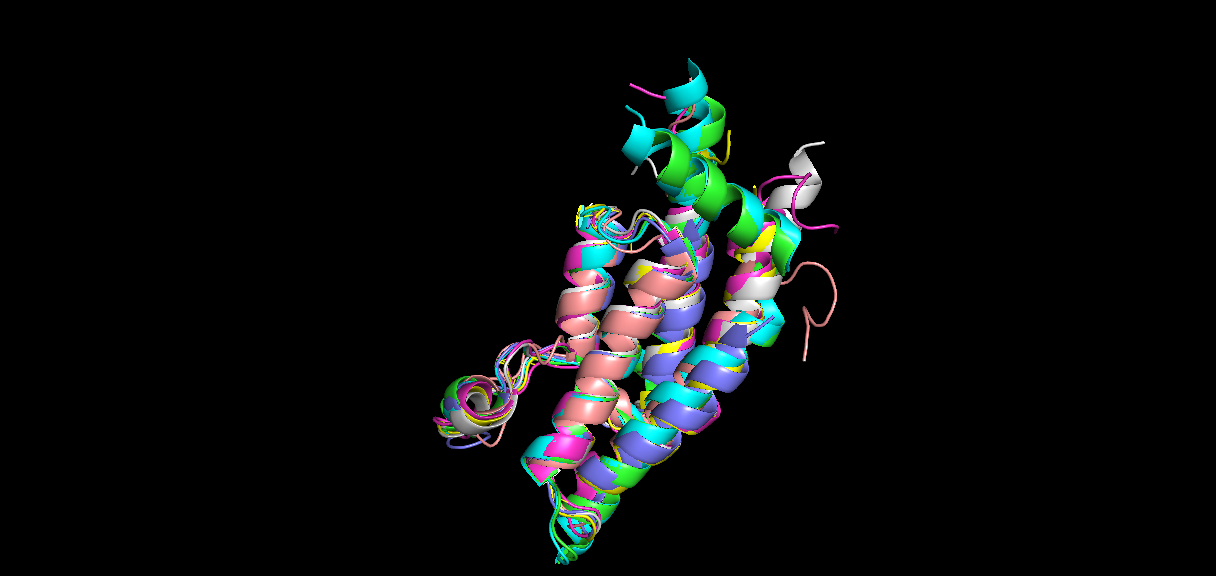
Family IV bromodomains include ATAD2, ATAD2B, BRPF1A, BRPF1B, BRPF3, BRD7, BRD1, and BRD9. When performing an alignment of each member of the Family IV bromodomains, there is distinct similarity in structure between them. The alignment was performed in PyMol and is shown in Figure 4. Given in Table 1 are the RMS values of this alignment, showing that the paralog, ATAD2B has the greatest similarity to the ATAD2 bromodomain and this is followed by BRD9 and so on.
Acetylated-Lysine Recognition
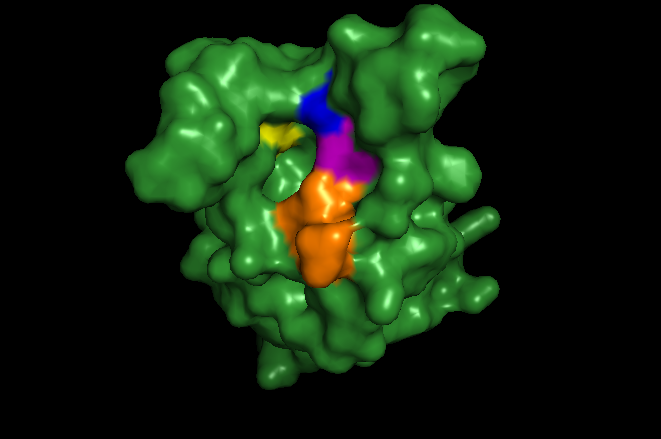
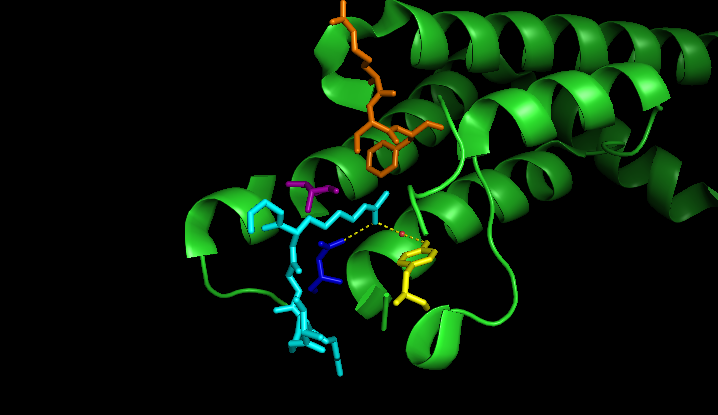
The ATAD2 bromodomain recognizes several acetylated lysines on the histone tail of H4. Among these are H4K5ac (PDBID: 4TT2), H4K12ac (PDBID: 4QUT), and more recently the diacetylated H4K5acK12ac (No structure deposited). The ATAD2 bromodomain also makes contact with H3K14ac (PDBID: 4TT4), but the crystal structure shows an interaction outside of the bromodomain binding pocket. Several residues are involved in ligand coordination. These include the conserved TYR1021, ASN1064, and the gatekeeper residue ILE1074. Also involved are members of the RVF shelf, ARG1007, VAL1008, and PHE1009 which provide additional selectivity for acetylated ligands alongside the gatekeeper residue. TYR1021 and ASN1064 are responsible for coordination of the ligands. Of note, TYR1021 interacts with ligands through a water molecule. Differences in these residues across bromodomains confer specificity of individual bromodomains. These residues are shown highlighted in Figure 3. RVF shelf is orange, conserved tyrosine is yellow, the conserved asparagine is blue, and the gatekeeper residue is shown in purple. This shows how the residues meet to form the binding pocket of the bromodomain. The specific connections are shown in Figure 5, of the ATAD2 structure (green) complexed with H4K5ac colored (cyan). Colors of individual residues are conserved from Figure 6.
Function of ATAD2
ATAD2 functions as a transcriptional co-regulator and chromatin remodeling protein, regulating chromatin structure and function. More recently, ATAD2 is proposed to act as a reader of newly synthesized diacetylated histone marks and to function as a cofactor of DNA polymerase to aid in replication. ATAD2 is required for gene response to androgens and responds to estrogen as well as being recruited by the active estrogen receptor-alpha (ERα). E2 factor genes (E2F) also require ATAD2 to function. Aside from mammals, little is known about its function in multicellular eukaryotes. Outside of its normal roles, ATAD2 is found upregulated in cancers and interacts with oncogenes MYC and ACTR (AIB1, SRC-3).
Findings in Cancer
ATAD2 has been found overexpressed in various types of cancer, including, but not limited to, breast, prostate, cervical, endometrial, lung, gastric, renal, and colorectal. ATAD2’s expression is amplified through a positive feedback loop in cancer. ATAD2 interacts with estrogen and androgen receptors, namely ERα, E2F, and AR, which in turn will increase ATAD2 expression to overexpress the gene. Cancers will activate this gene systematically. Correlations with poor prognosis and survival rates make ATAD2 a novel marker. This relation to cancer makes ATAD2, namely the bromodomain, which guides the rest of the protein to specific marks, a novel therapeutic target. There has been discussion though that simple inhibition of the bromodomain may not be enough to be a therapy. The destruction of the entire ATAD2 protein however, would theoretically be the solution.
ATAD2 bromodomain as a target for cancer therapy
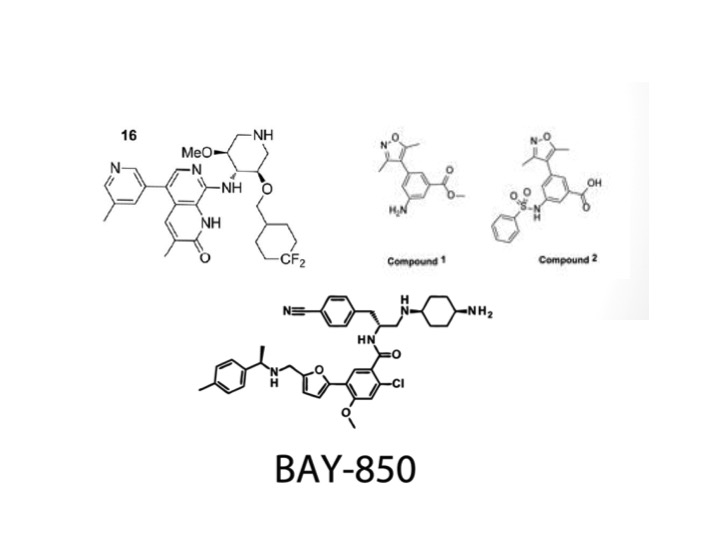
Several inhibitors have been designed over the last few years for the ATAD2 bromodomain, but there has been marginal success because of the bromodomain’s characterization as “difficult to drug.” Mentionable efforts have come from the MD Anderson cancer center and GlaxoSmithKline, which have produced compounds with sub 100nM potency and greater than 100-fold specificity over the BET bromodomains. There are still issues with non-selectivity and cell permeability, however. MD Anderson has produced two compounds (Compounds 1 and 2) that have an isoxazole base. GlaxoSmithKline has gone through a long refinement process to produce GSK8814 (Compound 16). This has a naphthyridone base and a replacement of a CF2 group for the sulfone found in the previous compound because it was not well tolerated in an area of lipophilic residues.
More recently, a group from Bayer has produced a new inhibitor that has a novel mechanism of action and structure in comparison to current inhibitors. BAY-850 comes from formyl acid derivatives, and induces dimerization of the protein to prevent interaction with acetylated histones.
There is hope for these compounds to lead to more specific and permeable drug candidates, but in the meantime they can be used as probes to determine ATAD2 function and involvement in cell systems. These compounds are shown in Figure 7.
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
</StructureSection>