User:Harish Srinivas
From Proteopedia
Revision as of 00:49, 6 December 2017
Sphingolipids together with glycerol-based phospholipids are major structural components of cell membranes. In response to various extracellular stimuli, including growth factors, inflammatory cytokines, antigens, and agonists of some GPCRs, the sphingolipids can be metabolized into potent mediators, such as Sphingosine-1-phosphate (S1P). This sphingolipid has emerged as an important signaling mediator participating in the regulation of multiple physiological and pathological processes taking place in cancer, cardiovascular diseases, wound healing, atherosclerosis and asthma but also is important in pathological conditions such as inflammation and stress. It can also trigger a range of biological effects such as cell migration, differentiation, apoptosis, immunity, proliferation and angiogenesis. The functioning of S1P receptors in the maintenance and modulation of the activity of the biological barrier is of the profound biological importance and has many therapeutic implications including treatment of multiple sclerosis, prevention of the transplant rejection and probably the adult respiratory distress syndrome as well.
|
Structural Features-
1.Transmembrane region:
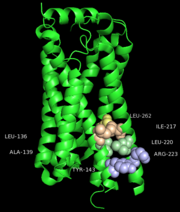
One cluster of core contacts links transmembrane (TM) I, II, and VII, cluster 1 consists of individual interaction groups. The second cluster of interactions links TM II, III, and IV through a series of four interaction groups and a frequently observed interaction between position interact through a hydrogen bond between an Asn or Ser and the indole nitrogen of a Trp. A third cluster of conserved contacts links together TM helices III, VI, and VII in the vicinity of the S1P1 receptor ligand binding pocket. These positions maintain important contacts between TM VI and TM III through two side chain-mediated interactions. Finally, cluster number four consists of three interactions that constrain TM V relative to TM III and one interaction between TM V and TM VI.
2.Exrtra-cellular region:
The extracellular region for all GPCRs consists of three loops: ECL1 between TM helices II and III, ECL2 between TM helices IV and V, and ECL3 between TM helices VI and VII. Optionally, there is a structured N-terminus that interacts with the ECLs. In the case of S1P1 receptor the structured N-terminus occludes the binding pocket, in the antagonist-bound state, cutting off access to the extracellular environment. One possible role for this structured N-terminus is that it is a feature of the S1P1 receptor structure in general and its presence implies the ligand does not access the binding pocket from the extracellular space directly. Instead, it is possible that the ligand gains access to the binding pocket through the lipid membrane where there is an enlarged gap between TM I and TM VI. This gap is larger in the S1P1 receptor than other class A GPCRs largely due to a shift in the position of the extracellular end of TM I away from TM VII in the S1P1 receptor.
3.Ligand binding region:
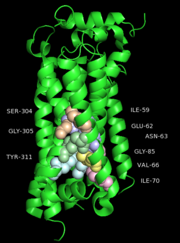
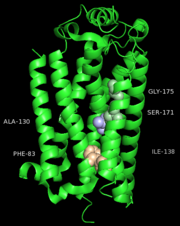
The S1P1 receptor provides 18 residues from the transmembrane region for interactions with the ML056 antagonist along with three additional residues from ECL2 and two from the N-terminus. ML056 lies in an amphipathic pocket where the head group phosphonate interactions are largely polar in nature and the alkyl chain tail interactions are largely hydrophobic as would be expected. The polar interactions observed for ML056 largely confirm mutagenesis data establishing the importance of Arg120 and Glu121 which were identified as important residues for supplying interactions with the zwitterionic sphingosine head group. In addition, the phosphonate head group of ML056 is surrounded by a ring of positively charged and polar residues contributed by TM helices III and VII, ECL2, and the N-terminal capping helix. Together these residues form a pocket that provides charge complementarity and high-affinity interactions to the phosphate group of the sphingolipids. A feature of ML056 is a primary amine located in the beta position relative to the phosphonate group. This primary amine is likely protonated and charged at physiological pH, thus enhancing its interactions with Glu121 through salt bridge formation. In addition to Glu121, Asn101 and Tyr98 provide hydrogen bonding interactions with the primary amine and amide linkage of ML056, respectively. The phenyl aryl tail of ML056 inserts into a hydrophobic pocket consisting of residues from TM helices III, V, VI, and VII, as well as ECL2. The pocket is lined with short aliphatic residues that define the shape and hydrophobicity of the pocket and four aromatic residues that provide the potential for specific interactions.
Lipid Receptor S1P1 Activation Scheme
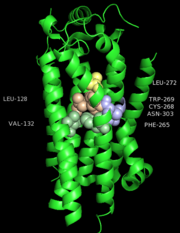
After binding of agonist S1P to the binding site of S1P1, the movement of acyl tail of S1P leads to the flipping of W269 (step 1). Such rotameric change alters the conformation of side chain of F265 which is located next to W269 in the same helix TM6 (step 2). These residues form a core of a transmission switch which involves rearrangement of centrally located residues including N63, D91, S304 and N307 They facilitate a redirected flow of water molecules inside a receptor (step 3). The influx of water molecules at intracellular part of the receptor leads to limited motions of cytoplasmic ends of TM helices, with the largest movement associated with TM7 (step 4), which is a prerequisite for larger motions of the cytoplasmic parts of transmembrane helices. These movements lead to opening the protein structure to make room for binding a G protein.
Other Features
S1P1 phosphorylation on S351, a residue crucial for receptor internalization. Impaired S1P1 phosphorylation enhances TH17 polarization and exacerbates autoimmune neuroinflammation. S1P1 receptor internalization is a critical step in initiating S1P signaling. This process is dependent on post-translational modification of the C-terminal domain of the receptor. Binding of S1P to S1P1 promotes the phosphorylation of C-terminal domain serine residues of S1P1 by protein kinase GRK2. This covalent addition of phosphate residue modifies the physicochemical properties of S1P1 leading to internalization of the ligandreceptor complex.