Mandelate racemase/muconate lactonizing enzyme
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
== Structural highlights == | == Structural highlights == | ||
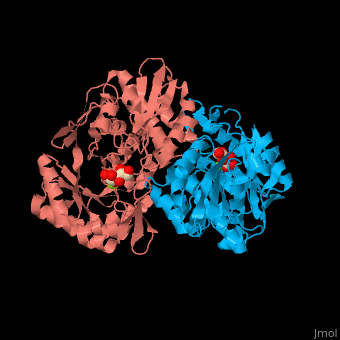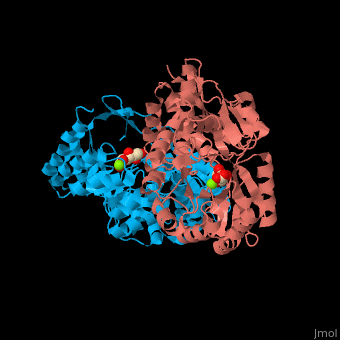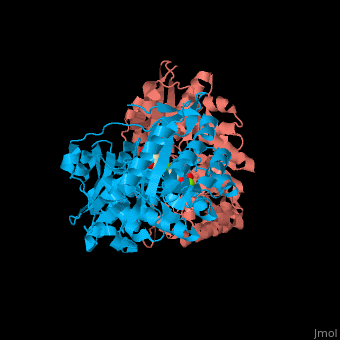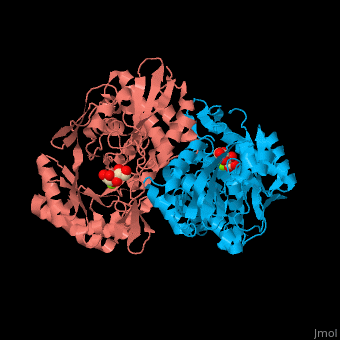
| - | The D-mannonate dehydratase has <scene name='77/775266/Cv/6'>two domains</scene>: the N-terminal α+β domain and a (β/α)<sub>7</sub> β-barrel domain. The <scene name='77/775266/Cv/4'>active site</scene> of the enzyme is found between these two domains<ref>PMID:179444491</ref>. <scene name='77/775266/Cv/5'>Mg coordination site</scene>. | + | The D-mannonate dehydratase has <scene name='77/775266/Cv/6'>two domains</scene>: the <scene name='77/775266/Cv/7'>N-terminal α+β domain</scene> and a <scene name='77/775266/Cv/8'>(β/α)<sub>7</sub> β-barrel domain</scene>. The <scene name='77/775266/Cv/4'>active site</scene> of the enzyme is found between these two domains<ref>PMID:179444491</ref>. <scene name='77/775266/Cv/5'>Mg coordination site</scene>. |
</StructureSection> | </StructureSection> | ||
Revision as of 13:23, 6 December 2017
| |||||||||||
3D Structures of Mandelate racemase/muconate lactonizing enzyme
Updated on 06-December-2017