We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Calreticulin
From Proteopedia
(Difference between revisions)
(New page: <StructureSection load='6eny' size='340' side='right' caption='Human calreticulin (gold) complex with β-microglobulin (green), tapasin (pink), protein disulfide-isomerase (yellow) MHC c...) |
|||
| Line 1: | Line 1: | ||
| - | |||
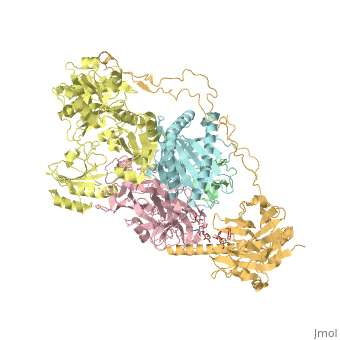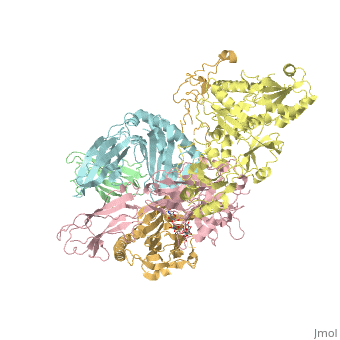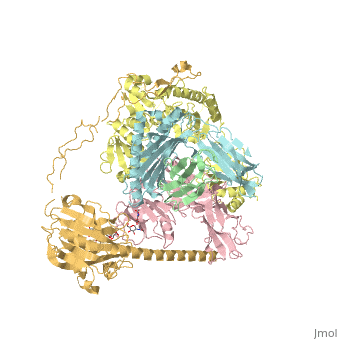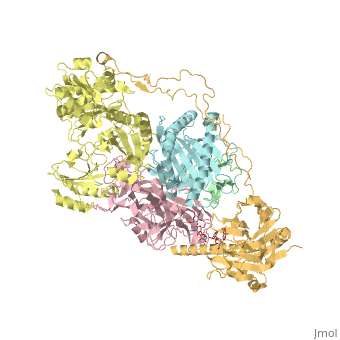
<StructureSection load='6eny' size='340' side='right' caption='Human calreticulin (gold) complex with β-microglobulin (green), tapasin (pink), protein disulfide-isomerase (yellow) MHC class I antigen (cyan) (PDB code [[6eny]]) ' scene=''> | <StructureSection load='6eny' size='340' side='right' caption='Human calreticulin (gold) complex with β-microglobulin (green), tapasin (pink), protein disulfide-isomerase (yellow) MHC class I antigen (cyan) (PDB code [[6eny]]) ' scene=''> | ||
| Line 17: | Line 16: | ||
== Structural highlights == | == Structural highlights == | ||
| - | CALR structure consists of 3 domains: N-terminal globular domain which has chaperone function; P-domain which is proline-rich, binds Ca+2 with high affinity and possesses a lectin-like chaperone function; C-terminal domain containing an ER retention signal. | + | <scene name='77/776393/Cv/1'>CALR structure consists of 3 domains</scene>: N-terminal globular domain which has chaperone function; P-domain which is proline-rich, binds Ca+2 with high affinity and possesses a lectin-like chaperone function; C-terminal domain containing an ER retention signal. |
</StructureSection> | </StructureSection> | ||
Revision as of 13:53, 29 January 2018
| |||||||||||
3D Structures of calreticulin
Updated on 29-January-2018
1hhn, 1k91, 1k9c – CALR P domain 189-288 – rat - NMR
6eny – hCALR + β-microglobulin + tapasin + protein disulfide-isomerase + MHC class I antigen – human
5v90 – hCALR P domain 238-273 + ERP29
3o0v, 3o0w, 3o0x, 3rg0 – CALR lectin domain 18-206 301-368 (mutant) – mouse
3pos, 3pow – hCALR lectin domain 18-206 301-368
5lk5 – hCALR lectin domain 18-206 301-368 (mutant)
5hca, 5hcb – CALR lectin domain 18-206 301-368 + glucose – Entamoeba histolytica
References
- ↑ Coppolino MG, Dedhar S. Calreticulin. Int J Biochem Cell Biol. 1998 May;30(5):553-8. PMID:9693955
- ↑ Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, Them NC, Berg T, Gisslinger B, Pietra D, Chen D, Vladimer GI, Bagienski K, Milanesi C, Casetti IC, Sant'Antonio E, Ferretti V, Elena C, Schischlik F, Cleary C, Six M, Schalling M, Schonegger A, Bock C, Malcovati L, Pascutto C, Superti-Furga G, Cazzola M, Kralovics R. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013 Dec 19;369(25):2379-90. doi: 10.1056/NEJMoa1311347. Epub 2013 , Dec 10. PMID:24325356 doi:http://dx.doi.org/10.1056/NEJMoa1311347
- ↑ Wang WA, Groenendyk J, Michalak M. Calreticulin signaling in health and disease. Int J Biochem Cell Biol. 2012 Jun;44(6):842-6. doi: 10.1016/j.biocel.2012.02.009., Epub 2012 Feb 21. PMID:22373697 doi:http://dx.doi.org/10.1016/j.biocel.2012.02.009