We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Polygalacturonase
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
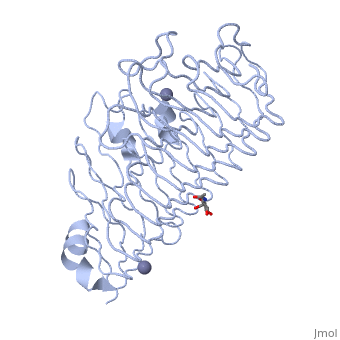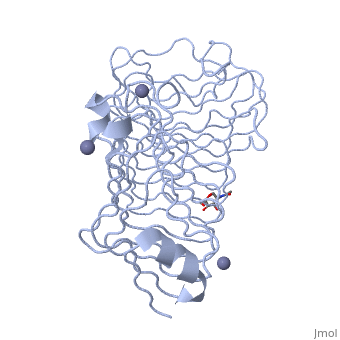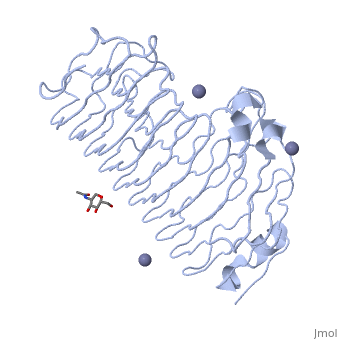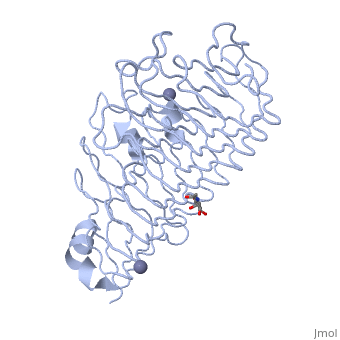
'''Polygalacturonases''' (PGs) catalyze the enzymatic depolymerization of pectates – polysaccharides that comprise the plant cell wall. Polymer disassembly of substrates by ''exo-'' and ''endo-'' PGs is carried out via a hydrolytic mechanism. Degradation of pectates in plant cell walls contributes to ripening of fruits, such as tomatoes and melons <ref>DOI: 10.1104/pp.117.2.337</ref>. Microbial PGs have been identified to be a part of defense mechanisms because of their role in pathogen attack <ref>DOI: 10.1074/jbc.273.38.24660</ref>. | '''Polygalacturonases''' (PGs) catalyze the enzymatic depolymerization of pectates – polysaccharides that comprise the plant cell wall. Polymer disassembly of substrates by ''exo-'' and ''endo-'' PGs is carried out via a hydrolytic mechanism. Degradation of pectates in plant cell walls contributes to ripening of fruits, such as tomatoes and melons <ref>DOI: 10.1104/pp.117.2.337</ref>. Microbial PGs have been identified to be a part of defense mechanisms because of their role in pathogen attack <ref>DOI: 10.1074/jbc.273.38.24660</ref>. | ||
| - | + | PG's are found in bacteria, fungi, plants, and animals. Plant PG's are involved in fruit ripening. Bacteria and fungal PG are involved in plant pathogenesis, often acting as plant virulence factors and involved in some of the initial pathogenic effects through their action of degrading the plant cell wall. | |
== Function == | == Function == | ||
| Line 8: | Line 8: | ||
Polygalacturonases hydrolyze α-(1-4) – glycosidic bonds between consecutive galacturonic acid residues in polygalacturonic acids. Structural variation has been identified among differing PGs depending on organismal origins and catalytic functions. For example, endo-polygalacturonases produced from <i>Erwinia carotovora </i> demonstrate functional similarity to pectate lyases in that they cleave polygalacturonic acids in a calcium-depended manner via β-elimination<ref>DOI: 10.1074/jbc.273.38.24660</ref>. | Polygalacturonases hydrolyze α-(1-4) – glycosidic bonds between consecutive galacturonic acid residues in polygalacturonic acids. Structural variation has been identified among differing PGs depending on organismal origins and catalytic functions. For example, endo-polygalacturonases produced from <i>Erwinia carotovora </i> demonstrate functional similarity to pectate lyases in that they cleave polygalacturonic acids in a calcium-depended manner via β-elimination<ref>DOI: 10.1074/jbc.273.38.24660</ref>. | ||
| - | + | One must distinguish between pectate and pectin. Pectate is a galacturonate polymer, pectin has a polygalacturonate backbone, but some of the monomers are methylesterified on the sixth carbon. PG acts on pectate, not pectin. | |
== Disease == | == Disease == | ||
Revision as of 19:12, 7 January 2018
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Joel L. Sussman, Krishna Amin, Michal Harel, Marilyn Yoder, OCA, Jaime Prilusky