We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Polygalacturonase
From Proteopedia
(Difference between revisions)
| Line 18: | Line 18: | ||
The tertiary fold of PGs varies in its composition of coils, approximating at 10 coils in a right-handed parallel beta helix domain along with loop regions that together form the substrate-binding cleft, which appears to have a tunnel-like shape. The active site of PGs is found between the looped regions of the protein. Located within the looped regions are two conserved aspartate residues that are predicted to participate in catalytic activity<ref name="crystal" />. | The tertiary fold of PGs varies in its composition of coils, approximating at 10 coils in a right-handed parallel beta helix domain along with loop regions that together form the substrate-binding cleft, which appears to have a tunnel-like shape. The active site of PGs is found between the looped regions of the protein. Located within the looped regions are two conserved aspartate residues that are predicted to participate in catalytic activity<ref name="crystal" />. | ||
| - | A right-handed parallel beta helix is a tertiary fold. The secondary structure is the beta and the alpha structure. If you describe how the secondary structure folds in space, that becomes tertiary structure. The secondary structural elements of the core fold of the proteins are only beta structure, the beta strands form parallel beta sheets. There are three main parallel beta sheets, PG's often have a smaller parallel beta sheet of only three-four beta strands. Nomenclature on how the sheets and turns are labeled are described in Yoder et al<ref>PMID:8081738</ref>. | + | A right-handed parallel beta helix is a tertiary fold. The secondary structure is the beta and the alpha structure. If you describe how the secondary structure folds in space, that becomes tertiary structure. The secondary structural elements of the core fold of the proteins are only beta structure, the beta strands form parallel beta sheets. There are three main parallel beta sheets, PG's often have a smaller parallel beta sheet of only three-four beta strands. Nomenclature on how the sheets and turns are labeled are described in Yoder et al<ref name="yoder">PMID:8081738</ref>. |
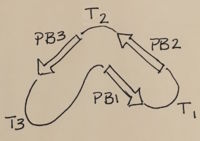
| - | Not all PGs have ten coils in the parallel beta helix. They usually have approximately 10 coils. [[Image:PBH2.jpg|200px|left|thumb| Nomenclature for structural elements of the parallel beta helix<ref | + | Not all PGs have ten coils in the parallel beta helix. They usually have approximately 10 coils. [[Image:PBH2.jpg|200px|left|thumb| Nomenclature for structural elements of the parallel beta helix<ref name="yoder" />. PB1 is Parallel Beta Sheet 1, T1 is Turn 1, between PB1 and PB2., [[PBH2]]]] With the parallel beta helix fold, the three major beta sheets are call PB1, PB2, and PB3. The turns between strands are Turn 1 (T1) between PB1 and PB2, T2 is the turn between PB2 and PB3, and T3 is the turn between PB3 and PB1 of the next coil. For your reference, this is illustrated in the figure below. |
== 3D Structures of polygalacturonase == | == 3D Structures of polygalacturonase == | ||
Revision as of 19:54, 7 January 2018
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Joel L. Sussman, Krishna Amin, Michal Harel, Marilyn Yoder, OCA, Jaime Prilusky