We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1329
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
== Relevance == | == Relevance == | ||
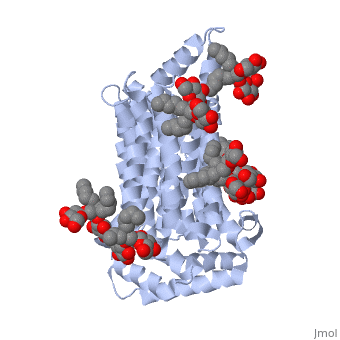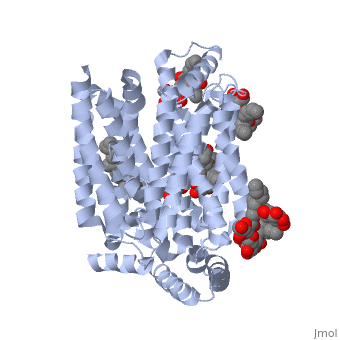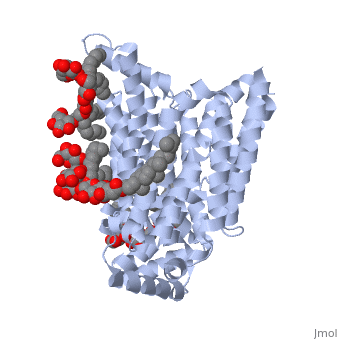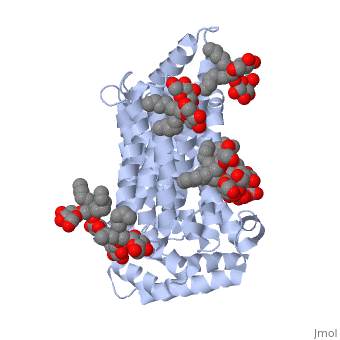
Both the amino and carbonyl termini of the protein are exposed on the cytoplasmic side of the plasma membrane. This protein functions via the <scene name='77/777649/Helices/1'>alternative confirmation model</scene>. A transport protein exposes a substrate towards either the outside or inside the cell. When the glucose or hexose binds to the site, it catalyzes a conformational change, releasing the glucose on the other side of the membrane. GLUT3 is a unique glucose transporter in that it functions even in times of low glucose. | Both the amino and carbonyl termini of the protein are exposed on the cytoplasmic side of the plasma membrane. This protein functions via the <scene name='77/777649/Helices/1'>alternative confirmation model</scene>. A transport protein exposes a substrate towards either the outside or inside the cell. When the glucose or hexose binds to the site, it catalyzes a conformational change, releasing the glucose on the other side of the membrane. GLUT3 is a unique glucose transporter in that it functions even in times of low glucose. | ||
| + | |||
== Structural highlights == | == Structural highlights == | ||
The protein contains 12 membrane-spanning alpha helices and has no known post-translational modifications. The first 6 transmembrane helices are in a pseudo symmetrical configuration relative to the last 6 helices. Helices 1, 2, 4, 5, 7, 8, 10, and 11 form an inner bundle that is stabilized by the outer helices 3, 6, 9, and 12. The GLUT3 protein is comprised of ~500 amino acid residues. It has a single site for N-Linked glycosylation, a central cytoplasmic linker domain, and exhibit topologies with their N and C termini, which are both positioned in the cytoplasm. | The protein contains 12 membrane-spanning alpha helices and has no known post-translational modifications. The first 6 transmembrane helices are in a pseudo symmetrical configuration relative to the last 6 helices. Helices 1, 2, 4, 5, 7, 8, 10, and 11 form an inner bundle that is stabilized by the outer helices 3, 6, 9, and 12. The GLUT3 protein is comprised of ~500 amino acid residues. It has a single site for N-Linked glycosylation, a central cytoplasmic linker domain, and exhibit topologies with their N and C termini, which are both positioned in the cytoplasm. | ||
| Line 19: | Line 20: | ||
| - | + | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
| + | https://www.ncbi.nlm.nih.gov/pubmed/19690067 | ||
| + | https://www.ncbi.nlm.nih.gov/books/NBK6545/ | ||
| + | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4104978/ | ||
| + | Carruthers A, DeZutter J, Ganguly A, Devaskar SU. Will the original glucose transporter isoform please stand up! Am J Physiol Endocrinol Metab. 2009;297(4):E836-848. | ||
| + | |||
<references/> | <references/> | ||
Revision as of 16:02, 27 February 2018
| This Sandbox is Reserved from January through July 31, 2018 for use in the course HLSC322: Principles of Genetics and Genomics taught by Genevieve Houston-Ludlam at the University of Maryland, College Park, USA. This reservation includes Sandbox Reserved 1311 through Sandbox Reserved 1430. |
To get started:
More help: Help:Editing |
Protein 5c65
| |||||||||||
References
https://www.ncbi.nlm.nih.gov/pubmed/19690067
https://www.ncbi.nlm.nih.gov/books/NBK6545/ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4104978/ Carruthers A, DeZutter J, Ganguly A, Devaskar SU. Will the original glucose transporter isoform please stand up! Am J Physiol Endocrinol Metab. 2009;297(4):E836-848.
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644