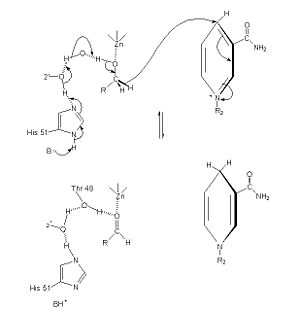
Alcohol dehydrogenase
From Proteopedia
(Difference between revisions)
| Line 23: | Line 23: | ||
<br/> | <br/> | ||
{{Clear}} | {{Clear}} | ||
| - | |||
| - | ==Structure== | ||
| - | |||
| - | The initial scene (<scene name='Birrer_Sandbox_2/Whole_adh_molecule/3'>Domains of ADH</scene>) shows an overview of the molecule, allowing for a general look at the tertiary structure of alcohol dehydrogenase (it is complexed with Cl, Pyz, NAD, and Zn). A second scene (<scene name='Birrer_Sandbox_2/Close_look_at_ligand/2'>Closer Look at Subunit</scene>) shows a close view of the ligand within each subunit. Labels have been placed on NAD, CL, and Zn to clearly establish the structure. | ||
| - | |||
| - | |||
| - | Within alcohol dehydrogenase, <scene name='Birrer_Sandbox_2/The_active_site/1'>the active</scene> site of alcohol dehydrogenase has three important residues, Phe 93, Leu 57, and Leu 116. These three residues work together to bind to the alcohol substrate.<ref>''Protein: Alcohol Dehydrogenase''. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://web.archive.org/web/20080307193453/http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm></ref> | ||
| - | |||
| - | |||
| - | Zn plays an important role in the catalysis. It funtions by electrostatically stabilizing the oxygen in alcohol during the reaction, which causes the alcohol to be more acidic. At the <scene name='Birrer_Sandbox_2/Zinc_binding_site/1'>Zinc Binding Site</scene>, Zinc coordinates with Cys 146, Cys 174, and His 67.<ref>''Protein: Alcohol Dehydrogenase''. The College of Saint Benedict and Saint John's University. 1 March 2010 <http://web.archive.org/web/20080307193453/http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm></ref> | ||
| - | |||
| - | |||
| - | NAD functions as a cosubstrate in the dehydration. NAD binds to numerous residues in a series of beta-alpha-beta folds. <scene name='Birrer_Sandbox_2/Nad_binding_site/1'>NAD Binding Region</scene> shows the domain where NAD binds, and many of the residues with which it interacts are selected. | ||
| - | <ref>''Protein: Alcohol Dehydrogenase''. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://web.archive.org/web/20080307193453/http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm</ref> | ||
| - | |||
| - | |||
| - | Alcohol dehydrogenase exists as a dimer with a zinc molecule complexed in each of the subunits. It has a SCOP catagory of an alpha and beta protein. At the N-terminal, there is a domain that is all beta; however, the C-Terminal domain is alpha and beta, so the catagory is alpha and beta. The C-Terminal core has 3 layers of alpha/beta/alpha and parallel beta sheets of 6 strands.<ref>''Protein: Alcohol dehydrogenase from Human (Homo sapiens), different isozymes''. SCOP. 2009. 1 March 2010 < http://web.archive.org/web/20060914235939/http://scop.berkeley.edu/data/scop.b.d.c.b.b.c.html></ref> | ||
| - | |||
==Reaction and Mechanism== | ==Reaction and Mechanism== | ||
Revision as of 11:24, 30 May 2018
| |||||||||||
Additional Resources
For additional information, see: Carbohydrate Metabolism
3D Structures of Alcohol dehydrogenase
Updated on 30-May-2018
References
- ↑ Voet, et. al. Fundamentals of Biochemistry: 3rd Edition. Hoboken: Wiley & Sons, Inc, 2008.
- ↑ Voet, et. al. Fundamentals of Biochemistry: 3rd Edition. Hoboken: Wiley & Sons, Inc, 2008.
- ↑ Protein: Alcohol Dehydrogenase. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://web.archive.org/web/20080307193453/http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm>
- ↑ Protein: Alcohol Dehydrogenase. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://web.archive.org/web/20080307193453/http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm>
- ↑ Voet, et. al. Fundamentals of Biochemistry: 3rd Edition. Hoboken: Wiley & Sons, Inc, 2008.
- ↑ Dickinson FM, Monger GP. A study of the kinetics and mechanism of yeast alcohol dehydrogenase with a variety of substrates. Biochem J. 1973 Feb;131(2):261-70. PMID:4352908
- ↑ Dickinson FM, Monger GP. A study of the kinetics and mechanism of yeast alcohol dehydrogenase with a variety of substrates. Biochem J. 1973 Feb;131(2):261-70. PMID:4352908
- ↑ Bille V, Remacle J. Simple-kinetic descriptions of alcohol dehydrogenase after immobilization on tresyl-chloride-activated agarose. Eur J Biochem. 1986 Oct 15;160(2):343-8. PMID:3769934
- ↑ Dickinson FM, Monger GP. A study of the kinetics and mechanism of yeast alcohol dehydrogenase with a variety of substrates. Biochem J. 1973 Feb;131(2):261-70. PMID:4352908
- ↑ Blomstrand R, Ostling-Wintzell H, Lof A, McMartin K, Tolf BR, Hedstrom KG. Pyrazoles as inhibitors of alcohol oxidation and as important tools in alcohol research: an approach to therapy against methanol poisoning. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3499-503. PMID:115004
- ↑ Alcohol Dehydrogenase. Worthington Biochemical Corporation . 31 March 2010 < http://http://www.worthington-biochem.com/ADH/default.html>
- ↑ Alcohol Dehydrogenase.Worthington Biochemical Corporation . 31 March 2010 < http://http://www.worthington-biochem.com/ADH/default.html>
- ↑ Goihberg E, Dym O, Tel-Or S, Levin I, Peretz M, Burstein Y. A single proline substitution is critical for the thermostabilization of Clostridium beijerinckii alcohol dehydrogenase. Proteins. 2007 Jan 1;66(1):196-204. PMID:17063493 doi:10.1002/prot.21170
- ↑ Goihberg E, Dym O, Tel-Or S, Shimon L, Frolow F, Peretz M, Burstein Y. Thermal stabilization of the protozoan Entamoeba histolytica alcohol dehydrogenase by a single proline substitution. Proteins. 2008 Feb 7;. PMID:18260103 doi:10.1002/prot.21946
- ↑ Goihberg E, Peretz M, Tel-Or S, Dym O, Shimon L, Frolow F, Burstein Y. Biochemical and Structural Properties of Chimeras Constructed by Exchange of Cofactor-Binding Domains in Alcohol Dehydrogenases from Thermophilic and Mesophilic Microorganisms. Biochemistry. 2010 Feb 9. PMID:20102159 doi:10.1021/bi901730x
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, David Canner, Joel L. Sussman, David Birrer