12-oxophytodienoate reductase
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
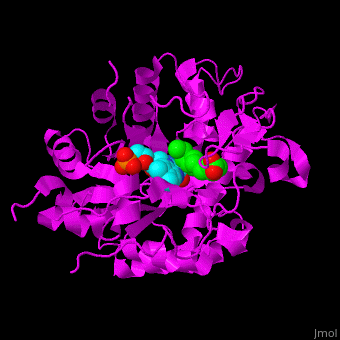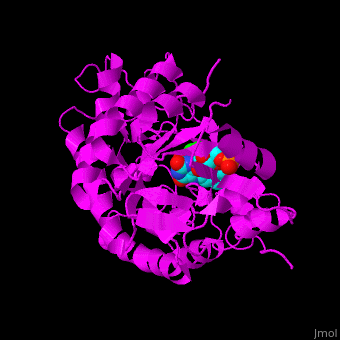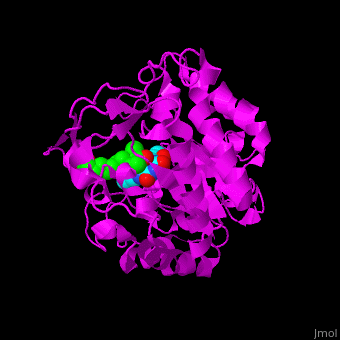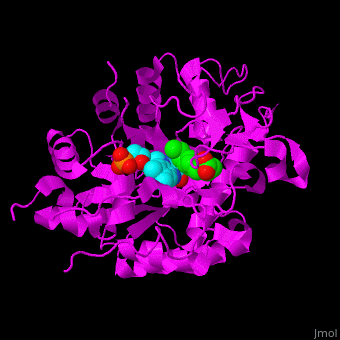
*<scene name='71/710043/Cv/16'>12-oxophytodienoate binding site</scene>. | *<scene name='71/710043/Cv/16'>12-oxophytodienoate binding site</scene>. | ||
*<scene name='71/710043/Cv/17'>FMN binding site</scene>. | *<scene name='71/710043/Cv/17'>FMN binding site</scene>. | ||
| - | *<scene name='71/710043/Cv/ | + | *<scene name='71/710043/Cv/15'>Click here to see whole active site</scene>. |
OPR exhibits self inhibition by dimerization.<ref>PMID:11377202</ref> Three isozymes of OPR are known – OPR1, OPR2, OPR3.<br /> | OPR exhibits self inhibition by dimerization.<ref>PMID:11377202</ref> Three isozymes of OPR are known – OPR1, OPR2, OPR3.<br /> | ||
* OPR1 cleaves olefinic bonds in α,β-unsaturated carbonyls.<br /> | * OPR1 cleaves olefinic bonds in α,β-unsaturated carbonyls.<br /> | ||
Revision as of 11:54, 21 March 2018
| |||||||||||
3D Structures of 12-oxophytodienoate reductase
Updated on 21-March-2018
References
- ↑ Breithaupt C, Strassner J, Breitinger U, Huber R, Macheroux P, Schaller A, Clausen T. X-ray structure of 12-oxophytodienoate reductase 1 provides structural insight into substrate binding and specificity within the family of OYE. Structure. 2001 May 9;9(5):419-29. PMID:11377202