Aspartate Aminotransferase
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
=='''Function'''== | =='''Function'''== | ||
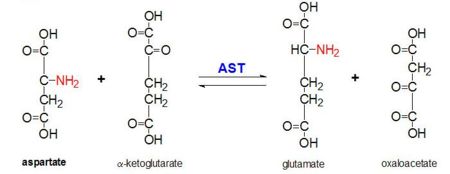
| - | [[Image:Ast-reaction final copy.JPG|right|thumb|upright=3|Figure 2: Transamination reaction of L-aspartate and α-ketoglutarate catalyzed by aspartate aminotransferase]] | + | [[Image:Ast-reaction final copy.JPG|right|thumb|450px|upright=3|Figure 2: Transamination reaction of L-aspartate and α-ketoglutarate catalyzed by aspartate aminotransferase]] |
{{Clear}} | {{Clear}} | ||
AAT catalyzes the reversible transamination of the α-amino group from L-aspartate to α-ketoglutarate forming oxaloacetate and glutamate<ref name ="AST ROLES AND STRUCTURE"/>. This reactivity is lower in E.coli than in higher eukaryotes, and has broader substrate specificity<ref name ="AST Structure"/>. However, the reaction takes place in the same way<ref name ="AST Structure"/>. Upon introduction of an amino acid substrate, a new Schiff base will form between it and the PLP cofactor<ref name ="AST Structure"/><ref name ="TRANSAMINATION">PMID:5450225</ref>. This causes the amino acid to lose a hydrogen and form a quinoid intermediate, and reprotanation takes place resulting in a ketimine<ref name ="AST Structure"/><ref name ="TRANSAMINATION"/>. Next, the structure is hydrolyzed forming an α-keto acid and pyridoxamine phosphate<ref name ="TRANSAMINATION"/>. 2-methyl aspartate acts as an inhibitor of AAT when it forms a Schiif base with the PLP cofactor, rather than aspartate<ref name ="TRANSAMINATION"/><ref name ="AST Structure"/>. This results in the process stopping at the step prior to the alpha protein elimination<ref name ="TRANSAMINATION"/><ref name ="AST Structure"/>. | AAT catalyzes the reversible transamination of the α-amino group from L-aspartate to α-ketoglutarate forming oxaloacetate and glutamate<ref name ="AST ROLES AND STRUCTURE"/>. This reactivity is lower in E.coli than in higher eukaryotes, and has broader substrate specificity<ref name ="AST Structure"/>. However, the reaction takes place in the same way<ref name ="AST Structure"/>. Upon introduction of an amino acid substrate, a new Schiff base will form between it and the PLP cofactor<ref name ="AST Structure"/><ref name ="TRANSAMINATION">PMID:5450225</ref>. This causes the amino acid to lose a hydrogen and form a quinoid intermediate, and reprotanation takes place resulting in a ketimine<ref name ="AST Structure"/><ref name ="TRANSAMINATION"/>. Next, the structure is hydrolyzed forming an α-keto acid and pyridoxamine phosphate<ref name ="TRANSAMINATION"/>. 2-methyl aspartate acts as an inhibitor of AAT when it forms a Schiif base with the PLP cofactor, rather than aspartate<ref name ="TRANSAMINATION"/><ref name ="AST Structure"/>. This results in the process stopping at the step prior to the alpha protein elimination<ref name ="TRANSAMINATION"/><ref name ="AST Structure"/>. | ||
Revision as of 12:29, 10 May 2018
| |||||||||||
3D structures of aspartate aminotransferase
Updated on 10-May-2018
References
- ↑ 1.0 1.1 Han Q, Robinson H, Cai T, Tagle DA, Li J. Biochemical and structural characterization of mouse mitochondrial aspartate aminotransferase, a newly identified kynurenine aminotransferase-IV. Biosci Rep. 2010 Oct 26. PMID:20977429 doi:10.1042/BSR20100117
- ↑ DeLorenzo RJ, Ruddle FH. Glutamate oxalate transaminase (GOT) genetics in Mus musculus: linkage, polymorphism, and phenotypes of the Got-2 and Got-1 loci. Biochem Genet. 1970 Apr;4(2):259-73. PMID:4193185
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Jeffery CJ, Gloss LM, Petsko GA, Ringe D. The role of residues outside the active site: structural basis for function of C191 mutants of Escherichia coli aspartate aminotransferase. Protein Eng. 2000 Feb;13(2):105-12. PMID:10708649
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 4.17 4.18 4.19 Kamitori S, Okamoto A, Hirotsu K, Higuchi T, Kuramitsu S, Kagamiyama H, Matsuura Y, Katsube Y. Three-dimensional structures of aspartate aminotransferase from Escherichia coli and its mutant enzyme at 2.5 A resolution. J Biochem. 1990 Aug;108(2):175-84. PMID:2121725
- ↑ Palaiologos G, Hertz L, Schousboe A. Role of aspartate aminotransferase and mitochondrial dicarboxylate transport for release of endogenously and exogenously supplied neurotransmitter in glutamatergic neurons. Neurochem Res. 1989 Apr;14(4):359-66. PMID:2569674
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 Tran A, Longo F, Ouzan D, Bianchi D, Pradier C, Saint-Paul MC, Sattonnet C, Laffont C, Dantin S, Piche T, Benzaken S, Rampal P. Effects of 1-year interferon-alpha 2a treatment in patients with chronic hepatitis C and persistently normal transaminase activity. Scand J Gastroenterol. 2000 Apr;35(4):433-7. PMID:10831269
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedAAT_Structure - ↑ 8.0 8.1 8.2 8.3 8.4 Martinez-Carrion M, Tiemeier DC, Peterson DL. Conformational properties of the isoenzymes of aspartate transaminase and the enzyme-substrate complexes. Biochemistry. 1970 Jun 23;9(13):2574-82. PMID:5450225
- ↑ 9.0 9.1 Tretter L, Adam-Vizi V. Inhibition of Krebs cycle enzymes by hydrogen peroxide: A key role of [alpha]-ketoglutarate dehydrogenase in limiting NADH production under oxidative stress. J Neurosci. 2000 Dec 15;20(24):8972-9. PMID:11124972
- ↑ 10.0 10.1 Tretter L, Adam-Vizi V. Inhibition of Krebs cycle enzymes by hydrogen peroxide: A key role of [alpha]-ketoglutarate dehydrogenase in limiting NADH production under oxidative stress. J Neurosci. 2000 Dec 15;20(24):8972-9. PMID:11124972
- ↑ 11.0 11.1 Jungas RL, Halperin ML, Brosnan JT. Quantitative analysis of amino acid oxidation and related gluconeogenesis in humans. Physiol Rev. 1992 Apr;72(2):419-48. PMID:1557428
- ↑ 12.0 12.1 Gibbs ME, Hertz L. Importance of glutamate-generating metabolic pathways for memory consolidation in chicks. J Neurosci Res. 2005 Jul 15;81(2):293-300. PMID:15929064 doi:10.1002/jnr.20548
- ↑ 13.0 13.1 13.2 13.3 13.4 Gonzalez-Flecha B, Cutrin JC, Boveris A. Time course and mechanism of oxidative stress and tissue damage in rat liver subjected to in vivo ischemia-reperfusion. J Clin Invest. 1993 Feb;91(2):456-64. PMID:8432855 doi:http://dx.doi.org/10.1172/JCI116223