We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Poly(A) binding protein
From Proteopedia
(Difference between revisions)
| Line 28: | Line 28: | ||
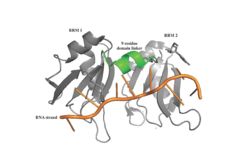
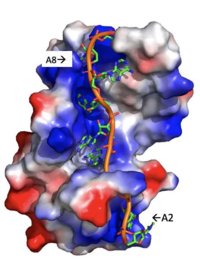
The initiation of translation in eukaryotes requires many translation factors and proteins, one of which is PABP. There is evidence that PABP is critical for formation of the “closed loop” model of protein synthesis, which involves joining the 3’ poly (A) tail of mRNA to the 5’ cap to create circular RNA. (B) This process utilizes eIF4F, a protein composed of multiple TFs that play various roles in translation. eIF4G is a scaffolding protein that binds the other subunits, eIF4E and eIF4A. eIF4E creates interactions with the 5’ cap to bring the IF complex to the 5’ end of the mRNA. eIF4A is an RNA helicase that denatures RNA and allows the ribosome to move along the strand. (C) All of these proteins are known to be involved in protein synthesis, but several mechanisms have been proposed for how eIF4G might also be interacting with PABP. | The initiation of translation in eukaryotes requires many translation factors and proteins, one of which is PABP. There is evidence that PABP is critical for formation of the “closed loop” model of protein synthesis, which involves joining the 3’ poly (A) tail of mRNA to the 5’ cap to create circular RNA. (B) This process utilizes eIF4F, a protein composed of multiple TFs that play various roles in translation. eIF4G is a scaffolding protein that binds the other subunits, eIF4E and eIF4A. eIF4E creates interactions with the 5’ cap to bring the IF complex to the 5’ end of the mRNA. eIF4A is an RNA helicase that denatures RNA and allows the ribosome to move along the strand. (C) All of these proteins are known to be involved in protein synthesis, but several mechanisms have been proposed for how eIF4G might also be interacting with PABP. | ||
| + | |||
| + | [[Image:closed loop.png|100 px|left|thumb|Figure Legend]] | ||
By observing protein synthesis in cells deficient of PABP, <ref name= "Kahvejian et al."> 7.Kahvejian, A. “Mammalian Poly(A)-Binding Protein Is a Eukaryotic Translation Initiation Factor, Which Acts via Multiple Mechanisms.” Genes & Development, vol. 19, no. 1, 2005, pp. 104–113., doi:10.1101/gad.1262905.</ref> were able to show that the PABP/eIF4G interaction promotes translation. The cells lacking PABP showed a seven-fold decrease in the rate of translation, which was remedied by reintroducing PABP to the cells. Other cells were treated with a PABP mutant that also had an eIF4G binding site, but the introduction of these proteins did not return the rate of translation to its normal level (C) These results show that not only is PABP acting as a TF in eukaryotic cells, but it also needs to interact with eIF4G in order to have an effect. | By observing protein synthesis in cells deficient of PABP, <ref name= "Kahvejian et al."> 7.Kahvejian, A. “Mammalian Poly(A)-Binding Protein Is a Eukaryotic Translation Initiation Factor, Which Acts via Multiple Mechanisms.” Genes & Development, vol. 19, no. 1, 2005, pp. 104–113., doi:10.1101/gad.1262905.</ref> were able to show that the PABP/eIF4G interaction promotes translation. The cells lacking PABP showed a seven-fold decrease in the rate of translation, which was remedied by reintroducing PABP to the cells. Other cells were treated with a PABP mutant that also had an eIF4G binding site, but the introduction of these proteins did not return the rate of translation to its normal level (C) These results show that not only is PABP acting as a TF in eukaryotic cells, but it also needs to interact with eIF4G in order to have an effect. | ||
Revision as of 18:20, 29 March 2018
Poly(A) binding protein
Structure
| |||||||||||