We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Poly(A) binding protein
From Proteopedia
(Difference between revisions)
| Line 18: | Line 18: | ||
[[Image: Stacking_of_adenosines_with_beta_sheets.jpg |250 px|left|thumb|Figure 2: The specific weak intermolecular interactions between RNP1 and RNP2 and Adenosines. These interactions are the primary support of adenosine recognition by PABP and include mainly van der Waals interactions, hydrogen bonds, and stacking interactions. ]] | [[Image: Stacking_of_adenosines_with_beta_sheets.jpg |250 px|left|thumb|Figure 2: The specific weak intermolecular interactions between RNP1 and RNP2 and Adenosines. These interactions are the primary support of adenosine recognition by PABP and include mainly van der Waals interactions, hydrogen bonds, and stacking interactions. ]] | ||
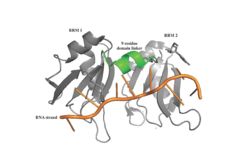
| - | A primary function of PABP is recognizing and interacting with the 3'poly (A) tail created in mRNA processing. As found by EMSA competition experiments, there is a minimum of 11-12 adenosines necessary in the poly (A) tail for the adenosine chain to bind to PABP with high affinity. However, for one biological assembly, a chain containing 9 adenosines sufficiently binds the assembly for crystallization and is shown in the biological assembly structure. The 4 RRM domains that are the primary interacting sites for the adenosine recognition exist as globular domains, each having four antiparallel β-strands and two α-helices. With the N-terminal to C-terminal motifs labeled as S1 to S4 for the β-strands and H1 to H2 for the α-helices, the strands are spatially arranged as S2-S3-S1-S4. Furthermore, there are two conserved sequences in each RRM, called RNP1 and 2. RNP 1 consists of a conserved sequence of 8 residues, while RNP2 consists of a conserved sequence of 6 residues. Much of the weak intermolecular interactions with adenosine from the RRMs occur from the <scene name='78/781946/Rnp1_rnp2_primary_a_binding/1'>RNP1 and RNP2</scene> conserved sequences, which correspond to the two central β-strands, with specific interactions shown in Figure 2.The support for adenosine recognition by the RRMs occurs as a type of binding trough with the sheets, primarily <scene name='78/781946/Rnp1_rnp2_primary_a_binding/1'>RNP1 and RNP2 forming the Primary Binding Trough</scene>, and the interstrand loop between β-strands 2 and 3 as well as the domain linker forming the <scene name='78/781946/Adenosine_binding_wall/1'> Adenosine Binding Wall</scene>. Additionally, the primary binding trough is stabilized by <scene name='78/781946/Rrm1_2_packing_intxn/2'>Stabilizing Packing Interactions of RRM1 RRM2 Binding Trough</scene>. | + | A primary function of PABP is recognizing and interacting with the 3'poly (A) tail created in mRNA processing. As found by EMSA competition experiments, there is a minimum of 11-12 adenosines necessary in the poly (A) tail for the adenosine chain to bind to PABP with high affinity. However, for one biological assembly, a chain containing 9 adenosines sufficiently binds the assembly for crystallization and is shown in the biological assembly structure. The 4 RRM domains that are the primary interacting sites for the adenosine recognition exist as globular domains, each having four antiparallel β-strands and two α-helices. With the N-terminal to C-terminal motifs labeled as S1 to S4 for the β-strands and H1 to H2 for the α-helices, the strands are spatially arranged as S2-S3-S1-S4. Furthermore, there are two conserved sequences in each RRM, called RNP1 and 2. RNP 1 consists of a conserved sequence of 8 residues, while RNP2 consists of a conserved sequence of 6 residues. Much of the weak intermolecular interactions with adenosine from the RRMs occur from the <scene name='78/781946/Rnp1_rnp2_primary_a_binding/1'>RNP1 and RNP2</scene> conserved sequences, which correspond to the two central β-strands, with specific interactions shown in Figure 2.The support for adenosine recognition by the RRMs occurs as a type of binding trough with the sheets, primarily <scene name='78/781946/Rnp1_rnp2_primary_a_binding/1'>RNP1 and RNP2 forming the Primary Binding Trough</scene>, and the interstrand loop between β-strands 2 and 3 as well as the domain linker forming the <scene name='78/781946/Adenosine_binding_wall/1'> Adenosine Binding Wall</scene>. Additionally, the primary binding trough is stabilized by <scene name='78/781946/Rrm1_2_packing_intxn/2'>Stabilizing Packing Interactions of RRM1 RRM2 Binding Trough</scene>. <ref name="PABP"> |
[[Image: Adenosine_backbone.png |200 px|right|thumb|Figure 3: Basic residues of RRM 1 and 2 (shown in blue) make stabilizing electrostatic interactions with the negatively charged adenosine phosphates. ]] | [[Image: Adenosine_backbone.png |200 px|right|thumb|Figure 3: Basic residues of RRM 1 and 2 (shown in blue) make stabilizing electrostatic interactions with the negatively charged adenosine phosphates. ]] | ||
| Line 27: | Line 27: | ||
===Translation Initiation=== | ===Translation Initiation=== | ||
| - | The initiation of translation in eukaryotes requires many translation factors and proteins, one of which is PABP. There is evidence that PABP is critical for formation of the “closed loop” model of protein synthesis, which involves joining the 3’ poly (A) tail of mRNA to the 5’ cap to create circular RNA. ( | + | The initiation of translation in eukaryotes requires many translation factors and proteins, one of which is PABP. There is evidence that PABP is critical for formation of the “closed loop” model of protein synthesis, which involves joining the 3’ poly (A) tail of mRNA to the 5’ cap to create circular RNA. <ref name= "Imataka">Imataka, H. “A Newly Identified N-Terminal Amino Acid Sequence of Human eIF4G Binds Poly(A)-Binding Protein and Functions in Poly(A)-Dependent Translation.” The EMBO Journal, vol. 17, no. 24, 1998, pp. 7480–7489., doi:10.1093/emboj/17.24.7480.</ref> This process utilizes eIF4F, a protein composed of multiple TFs that play various roles in translation. eIF4G is a scaffolding protein that binds the other subunits, eIF4E and eIF4A. eIF4E creates interactions with the 5’ cap to bring the IF complex to the 5’ end of the mRNA. eIF4A is an RNA helicase that denatures RNA and allows the ribosome to move along the strand. <ref name="kahvejian"/> All of these proteins are known to be involved in protein synthesis, but several mechanisms have been proposed for how eIF4G might also be interacting with PABP. |
| - | By observing protein synthesis in cells deficient of PABP, Kahvejian et. al <ref name="kahvejian">Kahvejian, A. “Mammalian Poly(A)-Binding Protein Is a Eukaryotic Translation Initiation Factor, Which Acts via Multiple Mechanisms.” Genes & Development, vol. 19, no. 1, 2005, pp. 104–113., doi:10.1101/gad.1262905</ref> were able to show that the PABP/eIF4G interaction promotes translation. The cells lacking PABP showed a seven-fold decrease in the rate of translation, which was remedied by reintroducing PABP to the cells. Other cells were treated with a PABP mutant that also had an eIF4G binding site, but the introduction of these proteins did not return the rate of translation to its normal level | + | By observing protein synthesis in cells deficient of PABP, Kahvejian et. al <ref name="kahvejian">Kahvejian, A. “Mammalian Poly(A)-Binding Protein Is a Eukaryotic Translation Initiation Factor, Which Acts via Multiple Mechanisms.” Genes & Development, vol. 19, no. 1, 2005, pp. 104–113., doi:10.1101/gad.1262905</ref> were able to show that the PABP/eIF4G interaction promotes translation. The cells lacking PABP showed a seven-fold decrease in the rate of translation, which was remedied by reintroducing PABP to the cells. Other cells were treated with a PABP mutant that also had an eIF4G binding site, but the introduction of these proteins did not return the rate of translation to its normal level <ref name="kahvejian"/> These results show that not only is PABP acting as a TF in eukaryotic cells, but it also needs to interact with eIF4G in order to have an effect. |
<scene name='78/781946/Pabp_linker_conserved_residues/1'>PABP linker with Conserved Residues Shown</scene> | <scene name='78/781946/Pabp_linker_conserved_residues/1'>PABP linker with Conserved Residues Shown</scene> | ||
| Line 39: | Line 39: | ||
== Medical Relevancy == | == Medical Relevancy == | ||
==='''Rotavirus' Effect on Initiation of Translation'''=== | ==='''Rotavirus' Effect on Initiation of Translation'''=== | ||
| - | The initiation of translation in eukaryotes is supported by a closed loop model. This model requires the 5' end and the 3' end of mRNA to be physically connected. The poly(A)-binding protein is necessary for initiation of translation and is required for the closed loop model. Rotavirus, a virus of varying size, containing 11 double stranded RNA and 12 proteins (6 structural, 6 non-structural) is responsible for preventing initiation of translation in infected cells. The virus enters the cell and undergoes a non-conservative replication cycle in the cytoplasm. After a replication cycle non-structural protein 3 (NSP3) can be found spread throughout the cytoplasm. NSP3 is responsible for releasing PABP from eIF4F and inhibiting translation initiation. In a study done by Piron et al. it has been seen that NSP3 competes with PABP in binding to the poly(A)-tail of mRNA. This competitor inhibits the proper closing of the closed loop therefore inhibiting translation and protein synthesis. Not only does the rotavirus inhibit protein synthesis of the host cell but it successfully initiatives its own translation as well. The viral mRNA and the host translation initiation factors are in close enough proximity to allow the viral mRNA bound to NSP3 to undergo translation. The translation of viral mRNA allows the virus to spread throughout an organism and lead to a greater decrease in host protein synthesis. When infected with rotavirus one may experience diarrhea, fever, vomiting, and dehydration. Without an antiviral it is suggested to increase fluid intake and allow three to seven days for the infection to subside. <ref> Piron, M. “Rotavirus RNA-Binding Protein NSP3 Interacts with eIF4GI and Evicts the Poly(A) Binding Protein from eIF4F.” The EMBO Journal, vol. 17, no. 19, 1998, pp. 5811–5821., doi:10.1093/emboj/17.19.5811. </ref> | + | The initiation of translation in eukaryotes is supported by a closed loop model. This model requires the 5' end and the 3' end of mRNA to be physically connected. The poly(A)-binding protein is necessary for initiation of translation and is required for the closed loop model. Rotavirus, a virus of varying size, containing 11 double stranded RNA and 12 proteins (6 structural, 6 non-structural) is responsible for preventing initiation of translation in infected cells. The virus enters the cell and undergoes a non-conservative replication cycle in the cytoplasm. After a replication cycle non-structural protein 3 (NSP3) can be found spread throughout the cytoplasm. NSP3 is responsible for releasing PABP from eIF4F and inhibiting translation initiation. In a study done by Piron et al. it has been seen that NSP3 competes with PABP in binding to the poly(A)-tail of mRNA. This competitor inhibits the proper closing of the closed loop therefore inhibiting translation and protein synthesis. Not only does the rotavirus inhibit protein synthesis of the host cell but it successfully initiatives its own translation as well. The viral mRNA and the host translation initiation factors are in close enough proximity to allow the viral mRNA bound to NSP3 to undergo translation. The translation of viral mRNA allows the virus to spread throughout an organism and lead to a greater decrease in host protein synthesis. When infected with rotavirus one may experience diarrhea, fever, vomiting, and dehydration. Without an antiviral it is suggested to increase fluid intake and allow three to seven days for the infection to subside. <ref name="Rotavirus"> Piron, M. “Rotavirus RNA-Binding Protein NSP3 Interacts with eIF4GI and Evicts the Poly(A) Binding Protein from eIF4F.” The EMBO Journal, vol. 17, no. 19, 1998, pp. 5811–5821., doi:10.1093/emboj/17.19.5811. </ref> |
== Biological Relevancy == | == Biological Relevancy == | ||
==='''Poly(A) Binding Protein's Evolution in plants'''=== | ==='''Poly(A) Binding Protein's Evolution in plants'''=== | ||
Revision as of 18:33, 29 March 2018
Poly(A) binding protein
Structure
| |||||||||||