We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Poly(A) binding protein
From Proteopedia
(Difference between revisions)
| Line 40: | Line 40: | ||
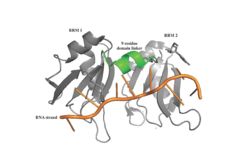
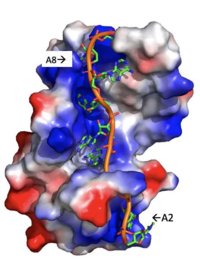
The RRMs support interactions with the interacting proteins such as eIF4G and [https://en.wikipedia.org/wiki/PAIP1 PAIP-1], however, the specific ways in which PABP interacts with these proteins are not structurally proven. However, there is a convex dorsal surface present on the RRM 1 and 2 motifs formed by the two α-helices in each RRM, specified as H1 and H2 in RRM1 and H1' and H2' in RRM2. This surface contains a sequence portion of <scene name='78/781947/H1_and_h2_h2ophobic_residues/2'>Conserved Hydrophobic Residues</scene>and <scene name='78/781947/Hydrophilic_residues/2'>Conserved Acidic Residues</scene> | The RRMs support interactions with the interacting proteins such as eIF4G and [https://en.wikipedia.org/wiki/PAIP1 PAIP-1], however, the specific ways in which PABP interacts with these proteins are not structurally proven. However, there is a convex dorsal surface present on the RRM 1 and 2 motifs formed by the two α-helices in each RRM, specified as H1 and H2 in RRM1 and H1' and H2' in RRM2. This surface contains a sequence portion of <scene name='78/781947/H1_and_h2_h2ophobic_residues/2'>Conserved Hydrophobic Residues</scene>and <scene name='78/781947/Hydrophilic_residues/2'>Conserved Acidic Residues</scene> | ||
| - | residues. It is thought that this area of conservation thus produces overlapping binding sites to interact with eIF4G and PAIP-1. For example, the conserved acidic residues may be beneficial to be used in to interact with essential basic residues present in both eIF4G and PAIP-1 via ionic interactions <ref name="PABP"/>. While there are only proposed mechanisms for how PABP promotes the initiation of translation in Homo sapiens, there is a pathogenic [https://en.wikipedia.org/wiki/Protozoa protozoan], Leishmania, that through a study done by Osvaldo P. de Melo Neto et. al, a site on the domain linker of a PABP homolog, PABP-1, of either serine-proline or threonine-proline residues which an eIF4F-like complex [https://en.wikipedia.org/wiki/Phosphorylation phosphorylates]. The authors suggest that this phosphorylation is part of how PABP-1 aids the eIF4F complex in initiating translation. They supported this by removing the gene that encodes PABP-1 and the results showed that the protozoan could not initiated cell growth and therefore survive without the PABP-1 gene. The homo sapiens' PABP also contain a | + | residues. It is thought that this area of conservation thus produces overlapping binding sites to interact with eIF4G and PAIP-1. For example, the conserved acidic residues may be beneficial to be used in to interact with essential basic residues present in both eIF4G and PAIP-1 via ionic interactions <ref name="PABP"/>. While there are only proposed mechanisms for how PABP promotes the initiation of translation in Homo sapiens, there is a pathogenic [https://en.wikipedia.org/wiki/Protozoa protozoan], Leishmania, that through a study done by Osvaldo P. de Melo Neto et. al, a site on the domain linker of a PABP homolog, PABP-1, of either serine-proline or threonine-proline residues which an eIF4F-like complex [https://en.wikipedia.org/wiki/Phosphorylation phosphorylates]. The authors suggest that this phosphorylation is part of how PABP-1 aids the eIF4F complex in initiating translation. They supported this by removing the gene that encodes PABP-1 and the results showed that the protozoan could not initiated cell growth and therefore survive without the PABP-1 gene. The homo sapiens' PABP also contain a (<scene name='78/781947/Pro-ser_in_linker/2'>Serine-Proline</scene>) site on the domain linker which could be interacting with the eIF4 complex in a similar way as in Leishmania protozoan.<ref name="Waldo"/> |
| Line 49: | Line 49: | ||
== Biological Relevancy == | == Biological Relevancy == | ||
==='''Poly(A) Binding Protein's Evolution in plants'''=== | ==='''Poly(A) Binding Protein's Evolution in plants'''=== | ||
| - | PABP is a conserved protein in eukaryotes and has been identified in yeast, mammals, and plants. While PABP is seen among these three it is only present in yeast as a single protein. [https://en.wikipedia.org/wiki/Mammal Mammalian] and plant PABP is in a diverse gene family and may have isoforms which have more specific functions. In a study done by Gallie and Liu it was seen that Arabidopsis, a small [https://en.wikipedia.org/wiki/Flowering_plant flowering plant], and yeast have multiple distinct PABP proteins which perform specific functions. The various PABP proteins in yeast vary in functions including acceleration of mRNA into a degradation pathway, mRNA biogenesis and export, and protecting the 5’ cap from degradation. While different species have different specific proteins the RRMs in PABP are highly conserved. The PABP has evolved overtime form a single gene found in yeast and algae to a large family of genes found in land plants and mammalian species. When PABP is viewed among different species of plants including Chlamydomonas reinhardtii, Chaetosphaeridium globosum, and Klebsormidium flaccidum it has been seen that different intron patterns are conserved in different species. The evolution of PABP has been seen among water plants, bryophytes, and [https://en.wikipedia.org/wiki/Vascular_plant vascular plants] as one of four classes of the gene. Class I genes are seen among certain basal angiosperms, monocots, gymnosperms, and bryophytes. Class II genes appear in seed plants while class III genes appear in specific charophytes and lycophytes. PABP evolved in plants from a single gene to two genes and eventually to a four-group gene family. This can be tracked with first marine algae then fresh water algae and finally [https://en.wikipedia.org/wiki/Non-vascular_plant non-vascular plants]. While the divergence of PABP gene families may impact the way protein interactions the PABP proteins all have similar functions. Gallie and Liu suggest that PABP gene families are still undergoing evolution. | + | PABP is a conserved protein in eukaryotes and has been identified in yeast, mammals, and plants. While PABP is seen among these three it is only present in yeast as a single protein. [https://en.wikipedia.org/wiki/Mammal Mammalian] and plant PABP is in a diverse gene family and may have isoforms which have more specific functions. In a study done by Gallie and Liu it was seen that Arabidopsis, a small [https://en.wikipedia.org/wiki/Flowering_plant flowering plant], and yeast have multiple distinct PABP proteins which perform specific functions. The various PABP proteins in yeast vary in functions including acceleration of mRNA into a degradation pathway, mRNA biogenesis and export, and protecting the 5’ cap from degradation. While different species have different specific proteins the RRMs in PABP are highly conserved. The PABP has evolved overtime form a single gene found in yeast and algae to a large family of genes found in land plants and mammalian species. When PABP is viewed among different species of plants including Chlamydomonas reinhardtii, Chaetosphaeridium globosum, and Klebsormidium flaccidum it has been seen that different intron patterns are conserved in different species. The evolution of PABP has been seen among water plants, bryophytes, and [https://en.wikipedia.org/wiki/Vascular_plant vascular plants] as one of four classes of the gene. Class I genes are seen among certain basal angiosperms, monocots, gymnosperms, and bryophytes. Class II genes appear in seed plants while class III genes appear in specific charophytes and lycophytes. PABP evolved in plants from a single gene to two genes and eventually to a four-group gene family. This can be tracked with first marine algae then fresh water algae and finally [https://en.wikipedia.org/wiki/Non-vascular_plant non-vascular plants]. While the divergence of PABP gene families may impact the way protein interactions the PABP proteins all have similar functions. Gallie and Liu suggest that PABP gene families are still undergoing evolution <ref name="gallie">Gallie, Daniel R, and Renyi Liu. “Phylogenetic Analysis Reveals Dynamic Evolution of the Poly(A)-Binding Protein Gene Family in Plants.” BMC Evolutionary Biology, vol. 14, no. 1, 2014, doi:10.1186/s12862-014-0238-4.</ref>. |
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 23:13, 29 March 2018
Poly(A) binding protein
Structure
| |||||||||||