We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Kyle Burton/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
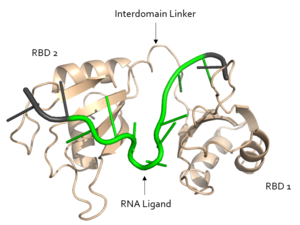
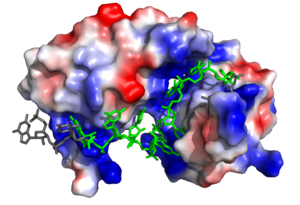
The ligand pre-mRNA sequence forms a <scene name='78/783145/U5_u6_u7_loop/5'>loop</scene> at U5, U6, and U7. This interaction is stabilized by π stacking between the G4 and <scene name='78/783145/Aromatic_stacking/4'>Y214</scene> as well as U5 and <scene name='78/783145/Aromatic_stacking/4'>F256</scene>, respectively<ref name="Handa"/>. The nucleobases are exposed to residues on Sxl due to the 2’ endo conformation of all the nucleotides except for U8, which maintains a 3’ endo conformation<ref name="Handa"/>. | The ligand pre-mRNA sequence forms a <scene name='78/783145/U5_u6_u7_loop/5'>loop</scene> at U5, U6, and U7. This interaction is stabilized by π stacking between the G4 and <scene name='78/783145/Aromatic_stacking/4'>Y214</scene> as well as U5 and <scene name='78/783145/Aromatic_stacking/4'>F256</scene>, respectively<ref name="Handa"/>. The nucleobases are exposed to residues on Sxl due to the 2’ endo conformation of all the nucleotides except for U8, which maintains a 3’ endo conformation<ref name="Handa"/>. | ||
| - | The U6 residue is recognized as part of the RNA <scene name='78/783145/U5_u6_u7_loop/13'>loop</scene> by R195. The R195 amide hydrogen-bonds to the O2' of U6 and the U6 N3H hydrogen bonds to the R195 carbonyl oxygen<ref name="Handa"/>. | + | The U6 residue is recognized as part of the RNA <scene name='78/783145/U5_u6_u7_loop/13'>loop at U5, U6, and U7</scene> by R195. The R195 amide hydrogen-bonds to the O2' of U6 and the U6 N3H hydrogen bonds to the R195 carbonyl oxygen<ref name="Handa"/>. |
| - | In the | + | In the <scene name='78/783145/U5_u6_u7_loop/2'>RNA loop</scene>, the U7 and U8 bases are involved in <scene name='78/783145/U7_u8_stacking/3'>π stacking</scene>, stabilizing the 3' endo conformation of the U8 sugar<ref name="Handa"/>. U8 is further stabilized via hydrogen bonding <scene name='78/783145/U8_with_s165_and_y166/3'>interactions with S165 and Y166</scene><ref name="Handa"/>. The amine group of U8 hydrogen bonds to the the carbonyl oxygens of both S165 and Y166 <ref name="Handa"/>. |
<scene name='78/783145/N130_interaction_with_u9/4'>U9</scene> is recognized by the interdomain linker <ref name="Handa"/>. This interaction is a [https://en.wikipedia.org/wiki/Salt_bridge salt bridge] between the N130 side chain and a phosphate oxygen of U9<ref name="Handa"/>. U9 is further stabilized by a second <scene name='78/783145/U9_with_interdomain_linker/1'>an ion-dipole interaction</scene> between the U9 O2' and the side chain of R202 and the U9 O4' and the K197 side chain<ref name="Handa"/>. | <scene name='78/783145/N130_interaction_with_u9/4'>U9</scene> is recognized by the interdomain linker <ref name="Handa"/>. This interaction is a [https://en.wikipedia.org/wiki/Salt_bridge salt bridge] between the N130 side chain and a phosphate oxygen of U9<ref name="Handa"/>. U9 is further stabilized by a second <scene name='78/783145/U9_with_interdomain_linker/1'>an ion-dipole interaction</scene> between the U9 O2' and the side chain of R202 and the U9 O4' and the K197 side chain<ref name="Handa"/>. | ||
Revision as of 17:49, 20 April 2018
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 Handa N, Nureki O, Kurimoto K, Kim I, Sakamoto H, Shimura Y, Muto Y, Yokoyama S. Structural basis for recognition of the tra mRNA precursor by the Sex-lethal protein. Nature. 1999 Apr 15;398(6728):579-85. PMID:10217141 doi:10.1038/19242
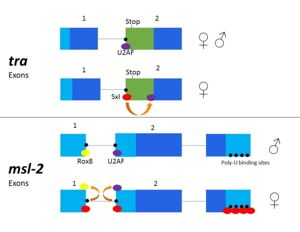
- ↑ 2.0 2.1 2.2 2.3 Penalva L, Sanchez L. RNA Binding Protein Sex-Lethal (Sxl) and Control of Drosophila Sex Determination and Dosage Compensation. Microbiol Mol Biol Rev.;67(3):343-356. doi: 10.1128/MMBR.67.3.343–359.2003
- ↑ 3.0 3.1 3.2 Bashaw GJ, Baker BS. The msl-2 dosage compensation gene of Drosophila encodes a putative DNA-binding protein whose expression is sex specifically regulated by Sex-lethal. Development. 1995 Oct;121(10):3245-58. PMID:7588059
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 Black DL. Mechanisms of alternative pre-messenger RNA splicing. Annu Rev Biochem. 2003;72:291-336. doi: 10.1146/annurev.biochem.72.121801.161720., Epub 2003 Feb 27. PMID:12626338 doi:http://dx.doi.org/10.1146/annurev.biochem.72.121801.161720
- ↑ 5.0 5.1 5.2 5.3 5.4 Georgiev P, Chlamydas S, Akhtar A. Drosophila dosage compensation: males are from Mars, females are from Venus. Fly (Austin). 2011 Apr-Jun;5(2):147-54. Epub 2011 Apr 1. PMID:21339706