We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Kyle Burton/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 26: | Line 26: | ||
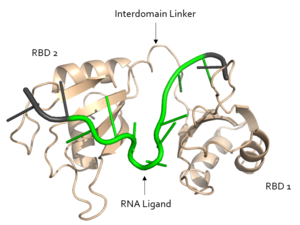
U11 is recognized by R155. The O2' of U11 <scene name='78/783145/R155_intxn_with_u11/3'>interacts with R155</scene> to form a hydrogen bond. | U11 is recognized by R155. The O2' of U11 <scene name='78/783145/R155_intxn_with_u11/3'>interacts with R155</scene> to form a hydrogen bond. | ||
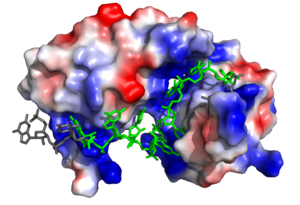
| - | The above interactions are relevant in that Sxl recognizes the specific pre-mRNA based mostly on interactions with the sugar-phosphate backbones<ref name="Handa"/>. Many proteins with [https://en.wikipedia.org/wiki/RNA_recognition_motif RNA recognition motifs] are specific in the interactions they form with the bases of the RNA recognized. In contrast, Sxl has a high specificity despite primarily interacting with the phosphate backbone. | + | The above interactions are relevant in that Sxl recognizes the specific pre-mRNA based mostly on interactions with the sugar-phosphate backbones<ref name="Handa"/>. Many proteins with [https://en.wikipedia.org/wiki/RNA_recognition_motif RNA recognition motifs] are specific in the interactions they form with the bases of the RNA recognized<ref name="Black"/>. In contrast, Sxl has a high specificity despite primarily interacting with the phosphate backbone. |
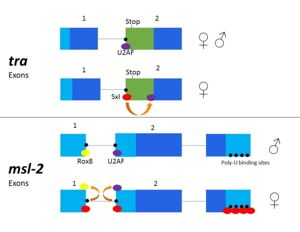
== Alternative Splicing Pathways == | == Alternative Splicing Pathways == | ||
| Line 42: | Line 42: | ||
== Relevance == | == Relevance == | ||
| - | As Sxl functions as a splicing repressor, it may give insight into the effects of varying mechanisms of alternate splicing both in flies and other species. Sxl may also lead to understanding of human alternative splicing factors | + | As Sxl functions as a splicing repressor, it may give insight into the effects of varying mechanisms of alternate splicing both in flies and other species. Sxl may also lead to understanding of human alternative splicing factors. As an RNA binding protein, research regarding Sxl may contribute to the understanding of enzymes with RNA recognition motifs. The Sxl RNP motif of RBD1 is also conserved in the ELAV family of proteins<ref name="Handa"/><ref>PMID: 9299339</ref>. Sxl is only one of many proteins which regulate dosage compensation, but is one that is observed in XX/XY systems, and thus could lead to better comprehension of dosage compensation in species with similar sex determination systems. |
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 23:30, 22 April 2018
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 Handa N, Nureki O, Kurimoto K, Kim I, Sakamoto H, Shimura Y, Muto Y, Yokoyama S. Structural basis for recognition of the tra mRNA precursor by the Sex-lethal protein. Nature. 1999 Apr 15;398(6728):579-85. PMID:10217141 doi:10.1038/19242
- ↑ 2.0 2.1 2.2 2.3 2.4 Penalva L, Sanchez L. RNA Binding Protein Sex-Lethal (Sxl) and Control of Drosophila Sex Determination and Dosage Compensation. Microbiol Mol Biol Rev.;67(3):343-356. doi: 10.1128/MMBR.67.3.343–359.2003
- ↑ 3.0 3.1 3.2 Bashaw GJ, Baker BS. The msl-2 dosage compensation gene of Drosophila encodes a putative DNA-binding protein whose expression is sex specifically regulated by Sex-lethal. Development. 1995 Oct;121(10):3245-58. PMID:7588059
- ↑ 4.0 4.1 4.2 Kelley RL, Solovyeva I, Lyman LM, Richman R, Solovyev V, Kuroda MI. Expression of msl-2 causes assembly of dosage compensation regulators on the X chromosomes and female lethality in Drosophila. Cell. 1995 Jun 16;81(6):867-77. PMID:7781064
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 Black DL. Mechanisms of alternative pre-messenger RNA splicing. Annu Rev Biochem. 2003;72:291-336. doi: 10.1146/annurev.biochem.72.121801.161720., Epub 2003 Feb 27. PMID:12626338 doi:http://dx.doi.org/10.1146/annurev.biochem.72.121801.161720
- ↑ 6.0 6.1 6.2 6.3 6.4 Georgiev P, Chlamydas S, Akhtar A. Drosophila dosage compensation: males are from Mars, females are from Venus. Fly (Austin). 2011 Apr-Jun;5(2):147-54. Epub 2011 Apr 1. PMID:21339706
- ↑ Lee AL, Volkman BF, Robertson SA, Rudner DZ, Barbash DA, Cline TW, Kanaar R, Rio DC, Wemmer DE. Chemical shift mapping of the RNA-binding interface of the multiple-RBD protein sex-lethal. Biochemistry. 1997 Nov 25;36(47):14306-17. doi: 10.1021/bi970830y. PMID:9398148 doi:http://dx.doi.org/10.1021/bi970830y
- ↑ 8.0 8.1 Bell LR, Horabin JI, Schedl P, Cline TW. Positive autoregulation of sex-lethal by alternative splicing maintains the female determined state in Drosophila. Cell. 1991 Apr 19;65(2):229-39. PMID:2015624
- ↑ Gebauer F, Merendino L, Hentze MW, Valcarcel J. The Drosophila splicing regulator sex-lethal directly inhibits translation of male-specific-lethal 2 mRNA. RNA. 1998 Feb;4(2):142-50. PMID:9570314
- ↑ Inoue M, Muto Y, Sakamoto H, Kigawa T, Takio K, Shimura Y, Yokoyama S. A characteristic arrangement of aromatic amino acid residues in the solution structure of the amino-terminal RNA-binding domain of Drosophila sex-lethal. J Mol Biol. 1997 Sep 12;272(1):82-94. PMID:9299339 doi:10.1006/jmbi.1997.1213