We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox GGC14
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
== Disease == | == Disease == | ||
| - | Defects in human a-Galactosidase gene can cause Fabry disease, a lysosomal storage disorder | + | Defects in the human a-Galactosidase gene can cause Fabry disease, a lysosomal storage disorder involving buildup of a-galactosylated substrates in tissues. It is an X-linked inherited disorder that affects 1 in 40,000 males. It is often characterized by chronic pain, vascular degeneration, cardiac anomalies, as well as other symptoms. The disease can show different levels of severity correlated with the amount of residual enzymatic activity. Organs affected can include the eyes, liver, kidney, and heart. More severe forms generally results from a complete loss of enzymatic activity, while milder phenotypes typically show some enzyme activity. Most people suffering from Fabry disease have a single point mutation in the gene, and over 400 missense and nonsense mutations have been identified. Other lysosomal storage disorder include the inherited diseases Tay-Sachs, Sandhoff, and Gaucher diseases. |
== Relevance == | == Relevance == | ||
| Line 29: | Line 29: | ||
[[Image:HBond_Pic2.png | thumb]] | [[Image:HBond_Pic2.png | thumb]] | ||
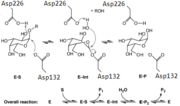
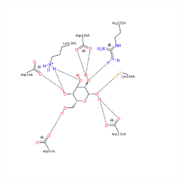
| - | The structure of the enzyme contains <scene name='78/781216/1t0o_alpha-galactosidase/8'>4 N-linked oligosaccharides</scene> comprised of 17 monosaccharides (15 monosaccharides while complexed with galactose). It also contains 2 domains, an <scene name='78/781216/1t0o_alpha-galactosidase/9'>N-terminal domain</scene> | + | The structure of the enzyme contains <scene name='78/781216/1t0o_alpha-galactosidase/8'>4 N-linked oligosaccharides</scene> comprised of 17 monosaccharides (15 monosaccharides while complexed with galactose). It also contains 2 domains, an <scene name='78/781216/1t0o_alpha-galactosidase/9'>N-terminal domain</scene> that can be described as having an a/B barrel topology, as well as a <scene name='78/781216/1t0o_alpha-galactosidase/10'>C-terminal domain</scene> that has an anti-parallel B-structure. |
Revision as of 13:00, 23 April 2018
1T0O - a-Galactosidase from Trichoderma reesei and Complex with Galactose
| |||||||||||
References
- ↑ Guce AI, Clark NE, Salgado EN, Ivanen DR, Kulminskaya AA, Brumer H 3rd, Garman SC. Catalytic mechanism of human alpha-galactosidase. J Biol Chem. 2010 Feb 5;285(6):3625-32. Epub 2009 Nov 25. PMID:19940122 doi:10.1074/jbc.M109.060145
- ↑ Golubev AM, Nagem RA, Brandao Neto JR, Neustroev KN, Eneyskaya EV, Kulminskaya AA, Shabalin KA, Savel'ev AN, Polikarpov I. Crystal structure of alpha-galactosidase from Trichoderma reesei and its complex with galactose: implications for catalytic mechanism. J Mol Biol. 2004 May 28;339(2):413-22. PMID:15136043 doi:http://dx.doi.org/10.1016/j.jmb.2004.03.062