We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Nuclear polyadenylated RNA-binding protein
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
==RBD-RBD Interactions and the Linker Region== | ==RBD-RBD Interactions and the Linker Region== | ||
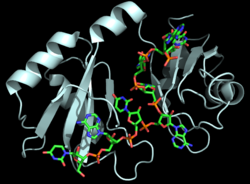
| - | As mentioned above, Hrp1 is composed of two RBDs. The RBDs are connected by a <scene name='78/783765/Linker/3'>linker region</scene> (a short two-turn α-helix), which also contains a crucial residue for RNA binding. Ile234 in the linker region holds Ade6 in place in order to ensure proper <scene name='78/781945/Linker_rna/1'>via van der Waals contacts</scene> with the nearby Phe162. Experimental evidence from protein [https://en.wikipedia.org/wiki/Nuclear_magnetic_resonance nuclear magnetic resonance (NMR)] data <ref name="GM3H"/> suggests that the two RBDs move independently prior to binding the PEE. Upon binding the PEE, the linker region adopts a short helical structure to rigidly hold the RBDs in place relative to each other. Aside from the linker helix, the only other interaction between the RBDs is | + | As mentioned above, Hrp1 is composed of two RBDs. The RBDs are connected by a <scene name='78/783765/Linker/3'>linker region</scene> (a short two-turn α-helix), which also contains a crucial residue for RNA binding. Ile234 in the linker region holds Ade6 in place in order to ensure proper <scene name='78/781945/Linker_rna/1'>via van der Waals contacts</scene> with the nearby Phe162. Experimental evidence from protein [https://en.wikipedia.org/wiki/Nuclear_magnetic_resonance nuclear magnetic resonance (NMR)] data <ref name="GM3H"/> suggests that the two RBDs move independently prior to binding the PEE. Upon binding the PEE, the linker region adopts a short helical structure to rigidly hold the RBDs in place relative to each other. Aside from the linker helix, the only other interaction between the RBDs is <scene name='78/781945/Interaction_between_domains/8'>a single salt bridge</scene> between Lys231 and Asp271 <ref name="GM3H"/>. |
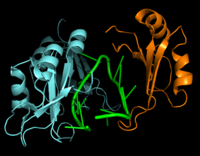
[[Image:Hrp1 RNA15 Cropped.png|200 px|left|thumb|Figure 2: Interaction between Hrp1 (blue), RNA15 (orange) and RNA (green).]] | [[Image:Hrp1 RNA15 Cropped.png|200 px|left|thumb|Figure 2: Interaction between Hrp1 (blue), RNA15 (orange) and RNA (green).]] | ||
Revision as of 22:26, 23 April 2018
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 Perez-Canadillas JM. Grabbing the message: structural basis of mRNA 3'UTR recognition by Hrp1. EMBO J. 2006 Jul 12;25(13):3167-78. Epub 2006 Jun 22. PMID:16794580
- ↑ 2.0 2.1 2.2 2.3 2.4 Leeper TC, Qu X, Lu C, Moore C, Varani G. Novel protein-protein contacts facilitate mRNA 3'-processing signal recognition by Rna15 and Hrp1. J Mol Biol. 2010 Aug 20;401(3):334-49. Epub 2010 Jun 19. PMID:20600122 doi:10.1016/j.jmb.2010.06.032
- ↑ Kessler MM, Henry MF, Shen E, Zhao J, Gross S, Silver PA, Moore CL. Hrp1, a sequence-specific RNA-binding protein that shuttles between the nucleus and the cytoplasm, is required for mRNA 3'-end formation in yeast. Genes Dev. 1997 Oct 1;11(19):2545-56. PMID:9334319
- ↑ Clery A, Blatter M, Allain FH. RNA recognition motifs: boring? Not quite. Curr Opin Struct Biol. 2008 Jun;18(3):290-8. doi: 10.1016/j.sbi.2008.04.002. PMID:18515081 doi:http://dx.doi.org/10.1016/j.sbi.2008.04.002
Proteopedia Page Contributors and Editors (what is this?)
Cory A. Wuerch, Matthew Douglas Moore, Savannah Davis, Michal Harel, Jaime Prilusky