We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Nuclear polyadenylated RNA-binding protein
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
=Structure= | =Structure= | ||
==General Features== | ==General Features== | ||
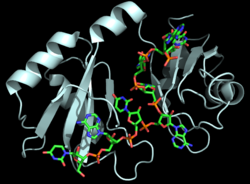
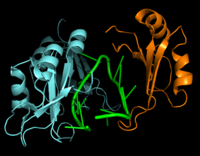
| - | Hrp1 is a single-stranded [https://en.wikipedia.org/wiki/RNA-binding_protein RNA-binding protein] composed of two RNP-type [https://en.wikipedia.org/wiki/RNA_recognition_motif RNA-binding domains (RBDs)] arranged in tandem with a typical ßαßßαß architecture <ref name="GM3H"/>. The two RBDs have similar topolgies, both containing a central [https://en.wikipedia.org/wiki/Beta_sheet antiparallel] four-stranded <scene name='78/783765/Beta_sheet/1'>ß-sheet</scene> with two [https://en.wikipedia.org/wiki/Alpha_helix α-helices] running across one face <ref name="GM3H"/>. The β-strands of each βαβ domain are linked via hydrogen bonding between conserved residues, <scene name='78/783765/L166_g201/4'>Leu166 and Gly201</scene>. The two RBDs associate to form a deep and positively charged <scene name='78/781960/Hrp1-rna_interface_surface/ | + | Hrp1 is a single-stranded [https://en.wikipedia.org/wiki/RNA-binding_protein RNA-binding protein] composed of two RNP-type [https://en.wikipedia.org/wiki/RNA_recognition_motif RNA-binding domains (RBDs)] arranged in tandem with a typical ßαßßαß architecture <ref name="GM3H"/>. The two RBDs have similar topolgies, both containing a central [https://en.wikipedia.org/wiki/Beta_sheet antiparallel] four-stranded <scene name='78/783765/Beta_sheet/1'>ß-sheet</scene> with two [https://en.wikipedia.org/wiki/Alpha_helix α-helices] running across one face <ref name="GM3H"/>. The β-strands of each βαβ domain are linked via hydrogen bonding between conserved residues, <scene name='78/783765/L166_g201/4'>Leu166 and Gly201</scene>. The two RBDs associate to form a deep and positively charged <scene name='78/781960/Hrp1-rna_interface_surface/3'>cleft</scene>, which constitutes the binding site for the RNA molecule <ref name="GM3H"/>. |
==Hrp1-RNA Interactions== | ==Hrp1-RNA Interactions== | ||
Revision as of 17:22, 24 April 2018
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 Perez-Canadillas JM. Grabbing the message: structural basis of mRNA 3'UTR recognition by Hrp1. EMBO J. 2006 Jul 12;25(13):3167-78. Epub 2006 Jun 22. PMID:16794580
- ↑ 2.0 2.1 2.2 2.3 2.4 Leeper TC, Qu X, Lu C, Moore C, Varani G. Novel protein-protein contacts facilitate mRNA 3'-processing signal recognition by Rna15 and Hrp1. J Mol Biol. 2010 Aug 20;401(3):334-49. Epub 2010 Jun 19. PMID:20600122 doi:10.1016/j.jmb.2010.06.032
- ↑ Kessler MM, Henry MF, Shen E, Zhao J, Gross S, Silver PA, Moore CL. Hrp1, a sequence-specific RNA-binding protein that shuttles between the nucleus and the cytoplasm, is required for mRNA 3'-end formation in yeast. Genes Dev. 1997 Oct 1;11(19):2545-56. PMID:9334319
- ↑ Clery A, Blatter M, Allain FH. RNA recognition motifs: boring? Not quite. Curr Opin Struct Biol. 2008 Jun;18(3):290-8. doi: 10.1016/j.sbi.2008.04.002. PMID:18515081 doi:http://dx.doi.org/10.1016/j.sbi.2008.04.002
Proteopedia Page Contributors and Editors (what is this?)
Cory A. Wuerch, Matthew Douglas Moore, Savannah Davis, Michal Harel, Jaime Prilusky