User:Alisa Cario
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
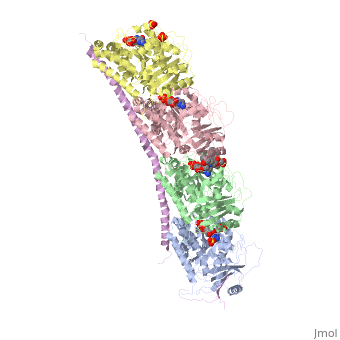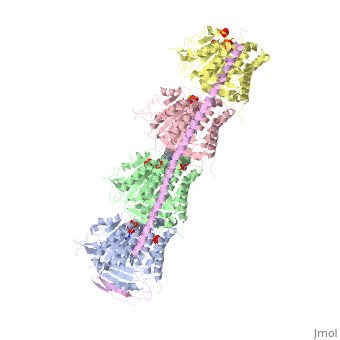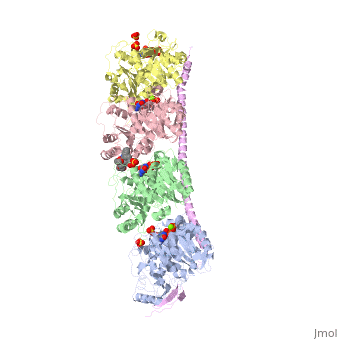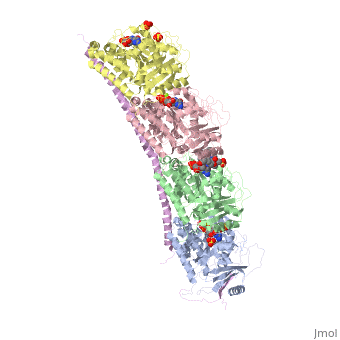
| - | == Stathmin-4 bound to Tubulin stabilized with Vinblastin == | + | == Stathmin-4 (RB3) bound to Tubulin stabilized with Vinblastin == |
[[4eb6]] | [[4eb6]] | ||
| Line 51: | Line 51: | ||
====How does the structure relate to it's function?==== | ====How does the structure relate to it's function?==== | ||
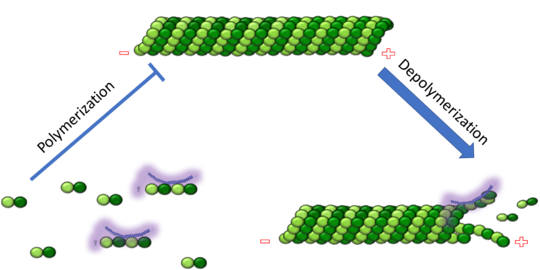
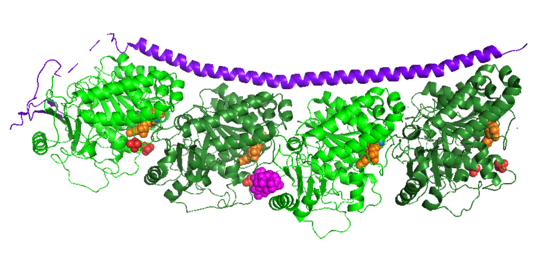
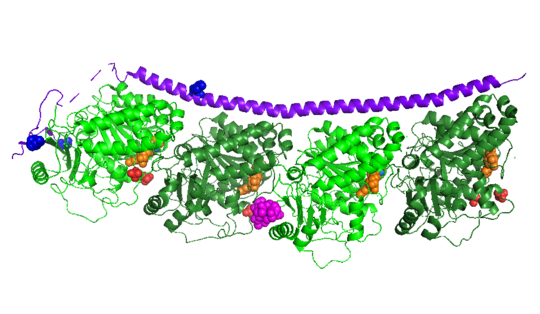
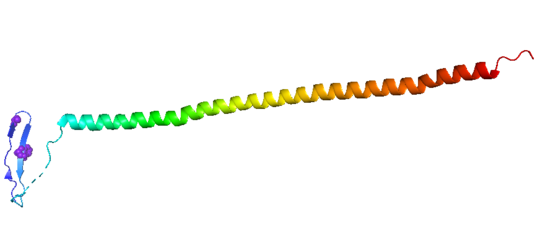
| - | Stathmin | + | Stathmin consistis of a coiled coil alpha helix, that binds across two tubulin heterodimers, and a mostly disordered N-terminal region that also has some beta strand properties. These different regions of the protein are known to have different functions. |
The N-terminal region is known to increase tubulin catastrophe. This region helps destabilize the ends of the microtubule filaments by curving the tubulin dimers at the end, and disrupting lateral hydrogen bonds. The N-terminal region is known as the regulatory domain of the protein, because it is subject to most of the post-translational modifications, as shown in Figure XXX. This region binds and caps alpha tubulin to accomplish this task. ( ) 4. | The N-terminal region is known to increase tubulin catastrophe. This region helps destabilize the ends of the microtubule filaments by curving the tubulin dimers at the end, and disrupting lateral hydrogen bonds. The N-terminal region is known as the regulatory domain of the protein, because it is subject to most of the post-translational modifications, as shown in Figure XXX. This region binds and caps alpha tubulin to accomplish this task. ( ) 4. | ||
| Line 63: | Line 63: | ||
== '''Disease relevance''' == | == '''Disease relevance''' == | ||
| - | '''Multiple Schlerosis''' | + | '''Multiple Schlerosis''': Stathmin expression is has been linked to multiple sclerosis (MS) , a neurodegenerative disorder characterized by lack of motor control and numbness. MS is caused by loss of myelination on axons in the nervous system. Myelination of axons is performed by cells called oligodendrocytes. Stathmin is regulated in oligodendrocyte lineage, with progenitor cells containing larger amounts than differentiated oligodendrocytes. Brain tissue samples from people suffering from multiple sclerosis found that stathmin is up regulated later in the lineage of oligodendrocytes. The up-regulation of stathmin showed a more globular morphology of the cells. Oligodendrocyte ability to myelinate axons in the central nervous system was greatly reduced in these patients (Reference). See [[Oligodendrocytes]] (REFERENCE). |
| - | Brain tissue samples from people suffering from multiple | + | |
| - | '''Cancer''' Due to stathmin's role in mitosis and cell migration, it is not surprising that is has been implicated in many cancers and is an active target of cancer therapeutics. Overexpression of stathmin has been shown to increase metastasis, worse prognosis, and increased chemoresistance ( ). Stathmin levels are known to be increasesd in a number of cancers. Stathmin was seen to be upregulated in breast cancer tissue comparative with normal breast tissue ( ). Another study was done to show stathmin upregulation in esophageal squamous cell carcinomas ( ). Studies using a non-phosphorylatable stathmin mutant shows that cells arrest during mitosis (Marklund, Larson) | + | '''Cancer''': Due to stathmin's role in mitosis and cell migration, it is not surprising that is has been implicated in many cancers and is an active target of cancer therapeutics. Stathmin is defined as an oncoprotein. Overexpression of stathmin has been shown to increase metastasis, worse prognosis, and increased chemoresistance ( ). Stathmin levels are known to be increasesd in a number of cancers. Stathmin was seen to be upregulated in breast cancer tissue comparative with normal breast tissue ( ). Another study was done to show stathmin upregulation in esophageal squamous cell carcinomas ( ). Studies using a non-phosphorylatable stathmin mutant shows that cells arrest during mitosis (Marklund, Larson) |
== '''Evolutionary conservation''' == | == '''Evolutionary conservation''' == | ||
| - | Stathmin belongs to a gene family that have a characteristic stathmin like domain. This family includes SCG10 | + | Stathmin belongs to a gene family that have a characteristic stathmin-like domain. There are four isoforms of stathmin which are alternatively spliced from a single gene. This family includes Stathmin-1, SCG10/stathmin-2, SCILP/stathmin-3, and RB3/stathmin-4. SCG10 and SCLIP, are exclusively neuronal proteins, while RB3 is expressed mostly in the brain but some in the adrenal glands. Unlike the neuronal proteins, stathmin-1 is ubiquitously expressed in all cell types. All members of this family contain the C-terminal coiled coil domain. However, the binding affinity to tubulin of the protein family members differ (. ). Interestingly, the neuronal isoforms are known to contain an additional N-terminal domain to affect their localization ( ). |
| - | '''Isoforms''' | ||
| - | There are four isoforms of stathmin which are alternatively spliced from a single gene in . Stathmin 1-3 are expressed early in development | ||
| - | DEVELOPMENTAL REGULATION | ||
| - | LOCATION OF ISOFORMS | ||
| - | HOW THEY DIFFER | ||
== Links to available structures == | == Links to available structures == | ||
Revision as of 16:47, 27 April 2018
* Full Real Name: Alisa Cario
- Position: Graduate Student
- Institution (NO ABBREVIATIONS): University of Vermont
- City, State/Province, Country: Burlington, VT USA
- Field of Expertise or Study: Creation of protopedia page for a class project. The class is Proteins 1 under Dr. Stephen Everse
Stathmin-4 (RB3) bound to Tubulin stabilized with Vinblastin
4eb6
| |||||||||||