Sandbox Reserved 1451
From Proteopedia
(Replacing page with '>{{Sandbox_Reserved_Telford2018}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> <StructureSection load='1jfp' size='340' side='right' caption='Bovine rhodopsin complex with retina...') |
|||
| Line 1: | Line 1: | ||
>{{Sandbox_Reserved_Telford2018}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | >{{Sandbox_Reserved_Telford2018}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
<StructureSection load='1jfp' size='340' side='right' caption='Bovine rhodopsin complex with retinal (PDB code [[1jfp]])'/> | <StructureSection load='1jfp' size='340' side='right' caption='Bovine rhodopsin complex with retinal (PDB code [[1jfp]])'/> | ||
| + | ==Rhodopsin== | ||
| + | |||
| + | Rhodopsin is a member of the G-protein coupled receptor (GPCR) family. Rhodopsin is the | ||
| + | common GPCR structure used to understand functionality of G-protein coupled receptors. | ||
| + | Rhodopsin is commonly found in the photoreceptors in the retina, specifically in the rod | ||
| + | photoreceptors and become activated by photons of light. Rhodopsin contains a chromophore | ||
| + | (compound that absorbs light), specifically 11-cis-retinal, which when active recruites G proteins | ||
| + | to transmit a signaling cascade in neural impulses to the gray matter of the occipital lobe. Once | ||
| + | the receptor has been activated, a new rhodopsin needs to be regenerated. Rhodopsin is | ||
| + | located in the rod outer segment (ROS) which consists of stacked disks enclosed by a | ||
| + | membrane. The entire family of GPCR’s have the common structure of seven alpha-helices | ||
| + | across membranes. Rhodopsin’s structure changes upon photoactivation. The sixth helix bends | ||
| + | away from the seventh creating a pocket that allows for binding of a G protein. A salt bridge | ||
| + | covers this pocket until the helices shift away from one another. Once the G protein has bound | ||
| + | then the signal can be transmitted to the occipital lobe. Over 120 point mutations to rhodopsin | ||
| + | have been identified which can lead to night blindness and more several visual problems<ref name="Article1">PMID:22266727</ref>. | ||
Revision as of 19:44, 30 April 2018
>
| This Sandbox is Reserved from Jan 22 through May 22, 2018 for use in the course Biochemistry II taught by Jason Telford at the Maryville University, St. Louis, Missouri, USA. This reservation includes Sandbox Reserved 1446 through Sandbox Reserved 1455. |
To get started:
More help: Help:Editing |
|
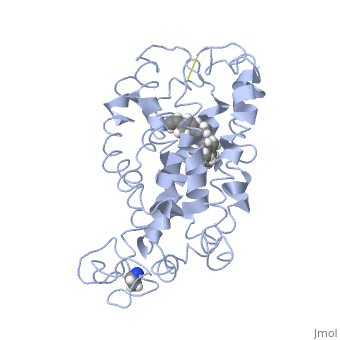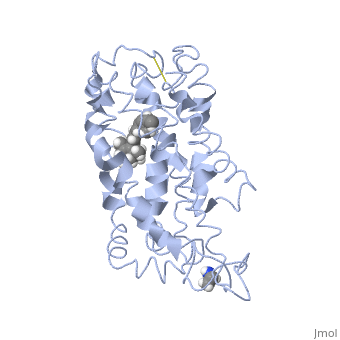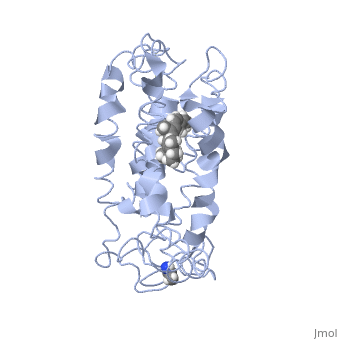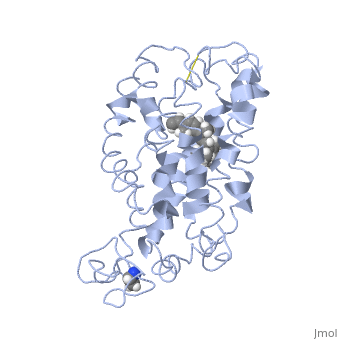
Rhodopsin
Rhodopsin is a member of the G-protein coupled receptor (GPCR) family. Rhodopsin is the common GPCR structure used to understand functionality of G-protein coupled receptors. Rhodopsin is commonly found in the photoreceptors in the retina, specifically in the rod photoreceptors and become activated by photons of light. Rhodopsin contains a chromophore (compound that absorbs light), specifically 11-cis-retinal, which when active recruites G proteins to transmit a signaling cascade in neural impulses to the gray matter of the occipital lobe. Once the receptor has been activated, a new rhodopsin needs to be regenerated. Rhodopsin is located in the rod outer segment (ROS) which consists of stacked disks enclosed by a membrane. The entire family of GPCR’s have the common structure of seven alpha-helices across membranes. Rhodopsin’s structure changes upon photoactivation. The sixth helix bends away from the seventh creating a pocket that allows for binding of a G protein. A salt bridge covers this pocket until the helices shift away from one another. Once the G protein has bound then the signal can be transmitted to the occipital lobe. Over 120 point mutations to rhodopsin have been identified which can lead to night blindness and more several visual problems[1].