User:Khadar Abdi/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
===N-terminus (Residue 1-241)=== | ===N-terminus (Residue 1-241)=== | ||
| - | The <scene name='78/786634/Tgs-domain/2'>N1-Domain</scene> and <scene name='78/786634/Editing_domain/1'>N-2 domain</scene> is the found in the N-terminus of the TARS enzyme chain. Specific N1 is found from 1-62 residue while N2 domain is found from 63-241 residue(PDB: [[ | + | The <scene name='78/786634/Tgs-domain/2'>N1-Domain</scene> and <scene name='78/786634/Editing_domain/1'>N-2 domain</scene> is the found in the N-terminus of the TARS enzyme chain. Specific N1 is found from 1-62 residue while N2 domain is found from 63-241 residue(PDB: [[1tje]]). Although located in neighboring regions, the N1-domain and N2-domain deviate dramatically from one another. Structurally, N1-domain has a TGS-fold (short for TARS, GTPase, Spo1 domain as their the most common proteins that present this fold)<ref>PMID:10447505</ref> which has 3 antiparallel beta sheets interacting with an alpha helix causing a formation of roll-like structure (similar to the description by Cath: Ubiquitin-like roll)<ref>http://www.rcsb.org/pdb/explore/macroMoleculeData.do?structureId=1TJE</ref>. The structure has no known function but it is pronounced structure found common within TARS. |
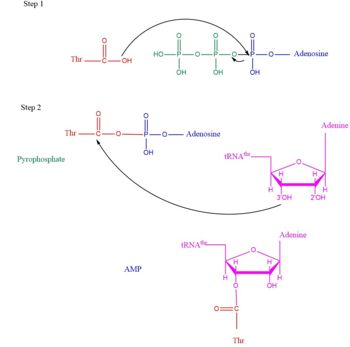
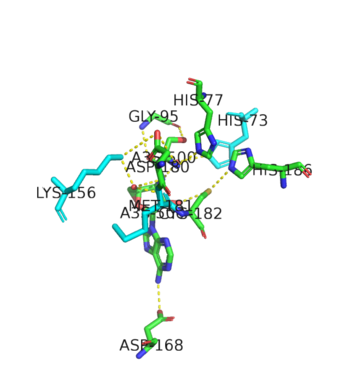
| - | This is the opposite of the N2-domain, or sometimes called TARS additional domain. This is ironic as this addition is very important for proofreading TARS activity<ref>PMID:15525511</ref>. TARS N2-domain is described as 2-layer alpha-beta sandwich comprised of mostly alpha helices. Interesting enough, the superfamily of this motif is found to be TARS and Alanyl-tRNA synthetase common domain. This domain function similar by discriminating Threonine and Alanine from Serine amino acid (Ser) as serine is similar structure to both amino acids. The editing domain functions first by moving Ser bound tRNA-Thr from the catalytic domain to the <scene name='78/786634/Editing_domain_residues/1'>editing site</scene> by breaking the bonds nucleotide A73, C74, and C75 from the catalytic site allowing the acceptor arm of tRNA-Thr to flip to the editing domain of S12-S13 site. Research on editing hydrolysis propose that residue Tyr 103 plays an important role of guiding acceptor arm towards the editing domain. The Ser bound to tRNA is hydrolzed by a water molecule, interacting with His73, acting as a nucleophile to the alpha carbon of serine, follow by protonation by a 2nd water molecule interacting with carbonyl of Met181 and amide side chain Lys156. The fidility mechanism of AA-tRNA binding is much similar to | + | This is the opposite of the N2-domain, or sometimes called TARS additional domain. This is ironic as this addition is very important for proofreading TARS activity<ref>PMID:15525511</ref>. TARS N2-domain is described as 2-layer alpha-beta sandwich comprised of mostly alpha helices. Interesting enough, the superfamily of this motif is found to be TARS and Alanyl-tRNA synthetase (AARS)common domain. This domain function similar by discriminating Threonine and Alanine from Serine amino acid (Ser) as serine is similar structure to both amino acids. The editing domain functions first by moving Ser bound tRNA-Thr from the catalytic domain to the <scene name='78/786634/Editing_domain_residues/1'>editing site</scene> by breaking the bonds nucleotide A73, C74, and C75 from the catalytic site allowing the acceptor arm of tRNA-Thr to flip to the editing domain of S12-S13 site. Research on editing hydrolysis propose that residue Tyr 103 plays an important role of guiding acceptor arm towards the editing domain. The Ser bound to tRNA is hydrolzed by a water molecule, interacting with His73, acting as a nucleophile to the alpha carbon of serine, follow by protonation by a 2nd water molecule interacting with carbonyl of Met181 and amide side chain Lys156. The specificity of serine binding to editing site versus threonine is due to steric hindrance by the presents of methyl group in the side chain. What is amazing about the fidility mechanism of AA-tRNA binding is the domain is much similar to (AARS) found in the C-terminus as they share 40% similarity in residues and both select for serine, although AARS also . The subtle difference of the isoforms mitochondrial TARS from cytoplasmic TARS appears in editing as the mitochondrial TARS doesn't have N2-domain and requires interaction to hydrolyze serine <ref>PMID:22773845</ref>. |
| + | |||
| + | [[Image:Editing domain.png|center|thumb|350px|'''Representative image of editing domain that demonstrates amino acid important in binding and hydrolyzing serine-tRNA(thr). Note, the amino acids highlighted in light blue are the amino acid important for hydrolysis (His-73, Met181, and Lys 156). Also not shown is Tyr103 who plays an important role in changing tRNA acceptor stem from catalytic domain to editing domain.''']] | ||
===Catalytic Domain (Residue 243-532)=== | ===Catalytic Domain (Residue 243-532)=== | ||
| - | As the name implies, the catalytic domain is the main area of protein activity. This domain is linked with N2-Domain of TARS by a alpha helix, or a linker helix (residue 225-242). The domain is architecturally described as a 2-layer alpha beta sandwhich. The importance of the domain is that it has 3 sites of binding: motif 1: ordering loop; motif 2 loop, Motif 3: threonine loop, and motif 4: ATP binding. The main important residues within in the | + | As the name implies, the catalytic domain is the main area of protein activity. This domain is linked with N2-Domain of TARS by a alpha helix, or a linker helix (residue 225-242). The domain is architecturally described as a 2-layer alpha beta sandwhich. The importance of the domain is that it has 3 sites of binding: motif 1: ordering loop; motif 2 loop, Motif 3: threonine loop, and motif 4: ATP binding. The main important residues within in the catalytic site include: Tyr ,Arg , Arg , His309 |
| Line 37: | Line 39: | ||
== Evolutionary related proteins == | == Evolutionary related proteins == | ||
| - | <scene name='78/786634/Tars_conservation/1'>Representation of TARS ''S. Aureus'' conservation of amino acids</scene> | + | |
| + | The image displays the <scene name='78/786634/Tars_conservation/1'>Representation of TARS ''S. Aureus'' conservation of amino acids</scene>. | ||
==Disease Relevance== | ==Disease Relevance== | ||
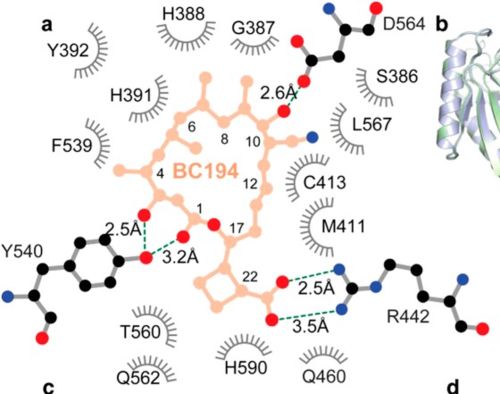
Lately the aaRS family was found to have more function than just aminoacylation. For instance, many aaRs molecules have been found to link with angiogenesis, blood vessel growth occuring within a cancer environment<ref>PMID:25535072</ref>. This is seen in human exogenous TARS in its ability to generate blood vessels within ovarian cancer environment<ref>PMID:23425968</ref>. Studies on the structure of TARS bound to BC194, derivative to the natural antibiotic Borrelidin, were investigated to understand how the angiogenic signaling from TARS occurs<ref>PMID: 26271225</ref>. | Lately the aaRS family was found to have more function than just aminoacylation. For instance, many aaRs molecules have been found to link with angiogenesis, blood vessel growth occuring within a cancer environment<ref>PMID:25535072</ref>. This is seen in human exogenous TARS in its ability to generate blood vessels within ovarian cancer environment<ref>PMID:23425968</ref>. Studies on the structure of TARS bound to BC194, derivative to the natural antibiotic Borrelidin, were investigated to understand how the angiogenic signaling from TARS occurs<ref>PMID: 26271225</ref>. | ||
| + | |||
| + | [[Image:Borrelidin binding.jpg|center|thumb|500px| '''Representative image of human TARS binding sites of BC194, TARS inhibitor. These sites show potential areas of where angiogenic activity of TARS. Note residue H388 and R442, amino acids that play important role catalytic activity, were found to interact with BC194, showing a potential of aminoacylation activity to possibly interconnect with angiogeneic activity. Not shown is TARS conformation change after presence of BC194 also a possible mechanism of inhibiting angiogeneic activity.''' <ref>PMID:26271225</ref>]] | ||
== List to available structures == | == List to available structures == | ||
===Bacterial=== | ===Bacterial=== | ||
| - | ''E. coli'': [[1qf6]] | + | ''E. coli'': [[1qf6]]Structure of ''E. coli'' TARS in the presence of tRNA |
| - | ''S. Aureus'': [[1nyq]] | + | ''S. Aureus'': [[1nyq]] |
Yeast (''Saccharomyces cerevisiae''): [[3ugt]] | Yeast (''Saccharomyces cerevisiae''): [[3ugt]] | ||
Revision as of 04:14, 2 May 2018
Threonyl-tRNA Synthetase/ligase
| |||||||||||