Alpha-tubulin N-acetyltransferase
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
'''Alpha-tubulin N-acetyltransferase''' (TAT) catalyzes the conversion of acetyl-CoA and tubulin-L-lysine to CoA and tubulin-N-acetyl-L-lysine. TAT acetylates Lys40 in tubulin and is required for normal sperm flagellar function. This posttranslational modification of tubulin is conserved in long-lived microtubules. TAT can acetylate itself and this acts as a regulatory mechanism for its action on tubulin.<ref>PMID:23071314</ref> | '''Alpha-tubulin N-acetyltransferase''' (TAT) catalyzes the conversion of acetyl-CoA and tubulin-L-lysine to CoA and tubulin-N-acetyl-L-lysine. TAT acetylates Lys40 in tubulin and is required for normal sperm flagellar function. This posttranslational modification of tubulin is conserved in long-lived microtubules. TAT can acetylate itself and this acts as a regulatory mechanism for its action on tubulin.<ref>PMID:23071314</ref> | ||
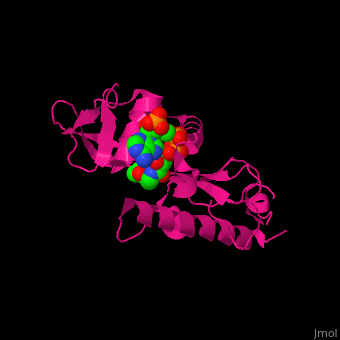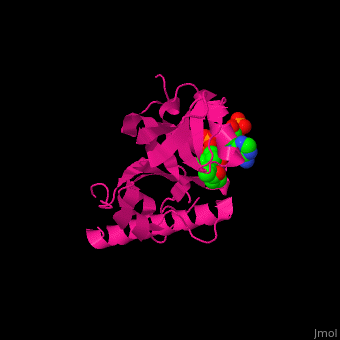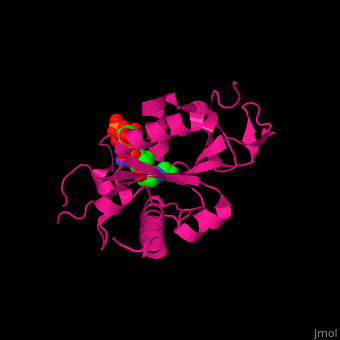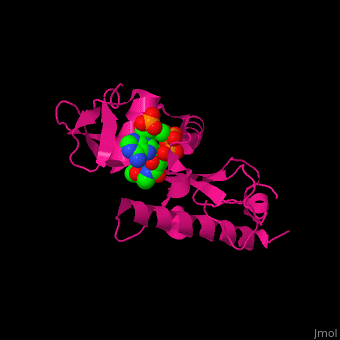
<scene name='70/707373/Cv/3'>CoA binding site</scene> of α-tubulin N-acetyltransferase (PDB code [[3vwe]]).<ref>PMID:20829795</ref> Water molecules are shown as red spheres. | <scene name='70/707373/Cv/3'>CoA binding site</scene> of α-tubulin N-acetyltransferase (PDB code [[3vwe]]).<ref>PMID:20829795</ref> Water molecules are shown as red spheres. | ||
| + | |||
| + | == 3D Structures of alpha-tubulin N-acetyltransferase == | ||
| + | [[Alpha-tubulin N-acetyltransferase 3D structures]] | ||
| + | |||
</StructureSection> | </StructureSection> | ||
Revision as of 09:28, 6 March 2019
| |||||||||||
3D Structures of Alpha-tubulin N-acetyltransferase
Updated on 06-March-2019
4gs4, 4b5o, 4if5 – hTAT catalytic domain - human
4b5p – hTAT catalytic domain (mutant)
4h6z, 4yrh – zfTAT catalytic domain – zebra fish
4h6u – zfTAT catalytic domain (mutant)
4hkf – zfTAT catalytic domain + acetyl-CoA
3vwd – hTAT catalytic domain + acetoacetyl-CoA
3vwe, 4u9y, 4u9z – hTAT catalytic domain + CoA
4pk2, 4pk3 – hTAT catalytic domain + bisubstrate analog
References
- ↑ Friedmann DR, Aguilar A, Fan J, Nachury MV, Marmorstein R. Structure of the alpha-tubulin acetyltransferase, alphaTAT1, and implications for tubulin-specific acetylation. Proc Natl Acad Sci U S A. 2012 Nov 27;109(48):19655-60. doi:, 10.1073/pnas.1209357109. Epub 2012 Oct 15. PMID:23071314 doi:10.1073/pnas.1209357109
- ↑ Akella JS, Wloga D, Kim J, Starostina NG, Lyons-Abbott S, Morrissette NS, Dougan ST, Kipreos ET, Gaertig J. MEC-17 is an alpha-tubulin acetyltransferase. Nature. 2010 Sep 9;467(7312):218-22. doi: 10.1038/nature09324. PMID:20829795 doi:10.1038/nature09324