We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Christian Fjeld/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 13: | Line 13: | ||
== Structure == | == Structure == | ||
| + | |||
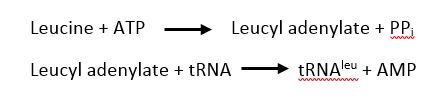
| + | LARS is composed of five domains, two catalyticly active domains; the catalytic and editing domains, one tRNA recognition domain; the anticodon binding domain, and two domains that pivot over the aminoacylation site; the leucine-specific and zinc binding domains. | ||
=== Catalytic Domain === | === Catalytic Domain === | ||
| Line 46: | Line 48: | ||
=== Zinc Binding Domain === | === Zinc Binding Domain === | ||
| - | The <scene name='78/786656/Zn1/1'>zinc binding domain</scene> is a highly variable domain and different species have have 0-2 zinc binding motifs within the domain<ref>doi: 10.1093/emboj/19.10.2351</ref>. It is highly disordered in the apo state and, similar to the leucine-specific domain, closes and interacts with the tRNA in the aminoacylation state. It is unclear if the zinc ligand actively participates in catalysis or is required for the correct folding of the domain | + | The <scene name='78/786656/Zn1/1'>zinc binding domain</scene> is a highly variable domain and different species have have 0-2 zinc binding motifs within the domain<ref>doi: 10.1093/emboj/19.10.2351</ref>. It is highly disordered in the apo state and, similar to the leucine-specific domain, closes and interacts with the tRNA in the aminoacylation state. It is unclear if the zinc ligand actively participates in catalysis or is required for the correct folding of the domain. Mutation of the zinc binding motif in some species, ''E. coli'', produces an inactive enzyme<ref>doi: 10.1093/emboj/19.10.2351</ref>. |
Revision as of 13:34, 3 May 2018
| |||||||||||
3D Structure of LARS
Updated on 03-May-2018
References
- ↑ Mirande M. The Aminoacyl-tRNA Synthetase Complex. Subcell Biochem. 2017;83:505-522. doi: 10.1007/978-3-319-46503-6_18. PMID:28271488 doi:http://dx.doi.org/10.1007/978-3-319-46503-6_18
- ↑ Han JM, Kim JY, Kim S. Molecular network and functional implications of macromolecular tRNA synthetase complex. Biochem Biophys Res Commun. 2003 Apr 18;303(4):985-93. doi: , 10.1016/s0006-291x(03)00485-6. PMID:12684031 doi:http://dx.doi.org/10.1016/s0006-291x(03)00485-6
- ↑ Raina M, Elgamal S, Santangelo TJ, Ibba M. Association of a multi-synthetase complex with translating ribosomes in the archaeon Thermococcus kodakarensis. FEBS Lett. 2012 Jul 30;586(16):2232-8. doi: 10.1016/j.febslet.2012.05.039. Epub, 2012 Jun 7. PMID:22683511 doi:http://dx.doi.org/10.1016/j.febslet.2012.05.039
- ↑ Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012 Apr 13;149(2):410-24. doi: 10.1016/j.cell.2012.02.044. Epub 2012 Mar, 15. PMID:22424946 doi:http://dx.doi.org/10.1016/j.cell.2012.02.044
- ↑ Seiradake E, Mao W, Hernandez V, Baker SJ, Plattner JJ, Alley MR, Cusack S. Crystal structures of the human and fungal cytosolic Leucyl-tRNA synthetase editing domains: A structural basis for the rational design of antifungal benzoxaboroles. J Mol Biol. 2009 Jul 10;390(2):196-207. Epub 2009 May 6. PMID:19426743 doi:10.1016/j.jmb.2009.04.073
- ↑ Palencia A, Crepin T, Vu MT, Lincecum TL Jr, Martinis SA, Cusack S. Structural dynamics of the aminoacylation and proofreading functional cycle of bacterial leucyl-tRNA synthetase. Nat Struct Mol Biol. 2012 Jun 10. doi: 10.1038/nsmb.2317. PMID:22683997 doi:10.1038/nsmb.2317
- ↑ Cusack S, Yaremchuk A, Tukalo M. The 2 A crystal structure of leucyl-tRNA synthetase and its complex with a leucyl-adenylate analogue. EMBO J. 2000 May 15;19(10):2351-61. PMID:10811626 doi:10.1093/emboj/19.10.2351
- ↑ doi: https://dx.doi.org/10.1016/S1097-2765(03)00098-4
- ↑ Seiradake E, Mao W, Hernandez V, Baker SJ, Plattner JJ, Alley MR, Cusack S. Crystal structures of the human and fungal cytosolic Leucyl-tRNA synthetase editing domains: A structural basis for the rational design of antifungal benzoxaboroles. J Mol Biol. 2009 Jul 10;390(2):196-207. Epub 2009 May 6. PMID:19426743 doi:10.1016/j.jmb.2009.04.073
- ↑ Seiradake E, Mao W, Hernandez V, Baker SJ, Plattner JJ, Alley MR, Cusack S. Crystal structures of the human and fungal cytosolic Leucyl-tRNA synthetase editing domains: A structural basis for the rational design of antifungal benzoxaboroles. J Mol Biol. 2009 Jul 10;390(2):196-207. Epub 2009 May 6. PMID:19426743 doi:10.1016/j.jmb.2009.04.073
- ↑ Liu Y, Liao J, Zhu B, Wang ED, Ding J. Crystal structures of the editing domain of Escherichia coli leucyl-tRNA synthetase and its complexes with Met and Ile reveal a lock-and-key mechanism for amino acid discrimination. Biochem J. 2006 Mar 1;394(Pt 2):399-407. PMID:16277600 doi:10.1042/BJ20051249
- ↑ Palencia A, Crepin T, Vu MT, Lincecum TL Jr, Martinis SA, Cusack S. Structural dynamics of the aminoacylation and proofreading functional cycle of bacterial leucyl-tRNA synthetase. Nat Struct Mol Biol. 2012 Jun 10. doi: 10.1038/nsmb.2317. PMID:22683997 doi:10.1038/nsmb.2317
- ↑ Palencia A, Crepin T, Vu MT, Lincecum TL Jr, Martinis SA, Cusack S. Structural dynamics of the aminoacylation and proofreading functional cycle of bacterial leucyl-tRNA synthetase. Nat Struct Mol Biol. 2012 Jun 10. doi: 10.1038/nsmb.2317. PMID:22683997 doi:10.1038/nsmb.2317
- ↑ Palencia A, Crepin T, Vu MT, Lincecum TL Jr, Martinis SA, Cusack S. Structural dynamics of the aminoacylation and proofreading functional cycle of bacterial leucyl-tRNA synthetase. Nat Struct Mol Biol. 2012 Jun 10. doi: 10.1038/nsmb.2317. PMID:22683997 doi:10.1038/nsmb.2317
- ↑ Cusack S, Yaremchuk A, Tukalo M. The 2 A crystal structure of leucyl-tRNA synthetase and its complex with a leucyl-adenylate analogue. EMBO J. 2000 May 15;19(10):2351-61. PMID:10811626 doi:10.1093/emboj/19.10.2351
- ↑ Cusack S, Yaremchuk A, Tukalo M. The 2 A crystal structure of leucyl-tRNA synthetase and its complex with a leucyl-adenylate analogue. EMBO J. 2000 May 15;19(10):2351-61. PMID:10811626 doi:10.1093/emboj/19.10.2351
- ↑ Palencia A, Crepin T, Vu MT, Lincecum TL Jr, Martinis SA, Cusack S. Structural dynamics of the aminoacylation and proofreading functional cycle of bacterial leucyl-tRNA synthetase. Nat Struct Mol Biol. 2012 Jun 10. doi: 10.1038/nsmb.2317. PMID:22683997 doi:10.1038/nsmb.2317