We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Christian Fjeld/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
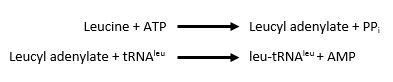
The <scene name='78/786656/Catalytic/1'>catalytic domain</scene> is responsible for the two step process of charging leucine on to tRNA<sup>leu</sup>. First, ATP and leucine are bound and AMP is transfered to the backbone carboxylic acid of leucine with the release of a pyrophosphate. Second, tRNA<sup>leu</sup> is bound with the leucyl adenylate and leucine is transfered to either the 2' OH of the 3' terminal adenine with the release of AMP<ref>doi: 10.1038/nsmb.2317</ref>. | The <scene name='78/786656/Catalytic/1'>catalytic domain</scene> is responsible for the two step process of charging leucine on to tRNA<sup>leu</sup>. First, ATP and leucine are bound and AMP is transfered to the backbone carboxylic acid of leucine with the release of a pyrophosphate. Second, tRNA<sup>leu</sup> is bound with the leucyl adenylate and leucine is transfered to either the 2' OH of the 3' terminal adenine with the release of AMP<ref>doi: 10.1038/nsmb.2317</ref>. | ||
| - | [[Image: | + | [[Image:Scheme2.JPG]] |
LARS, like all class I synthetases, is characterized by a Rossmann-fold catalytic domain with a central parallel β-sheet with α-helices on both faces. The active site catalyzes both the formation of the leucyl adenylate intermediate and the subsequent charging of leucine onto the terminal acceptor arm of the tRNA<ref>doi: 10.1093/emboj/19.10.2351</ref>. The binding pocket accommodates leucine as well as smaller amino acids such as isoleucine and valine. This allows tRNA<sup>leu</sup> to be mischarged with noncognate amino acids and must be corrected to ensure translational fidelity<ref>doi: 10.1016/S1097-2765(03)00098-4</ref>. | LARS, like all class I synthetases, is characterized by a Rossmann-fold catalytic domain with a central parallel β-sheet with α-helices on both faces. The active site catalyzes both the formation of the leucyl adenylate intermediate and the subsequent charging of leucine onto the terminal acceptor arm of the tRNA<ref>doi: 10.1093/emboj/19.10.2351</ref>. The binding pocket accommodates leucine as well as smaller amino acids such as isoleucine and valine. This allows tRNA<sup>leu</sup> to be mischarged with noncognate amino acids and must be corrected to ensure translational fidelity<ref>doi: 10.1016/S1097-2765(03)00098-4</ref>. | ||
| Line 28: | Line 28: | ||
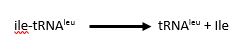
The <scene name='78/786656/Editing/1'>editing domain</scene> of LARS is responsible for hydrolysing mischarged tRNA, such as ile-tRNA<sup>leu</sup>, and releasing it for a subsequent round of aminoacylation. This editing function allows cells to achieve a tRNA charging error rate of less than 1:10,000<ref>doi: 10.1016/j.jmb.2009.04.073</ref>. The domain itself is an β sandwich connected to the catalytic domain by a flexible loop that allows a hinge action to bind aminoacylated tRNAs for error checking. | The <scene name='78/786656/Editing/1'>editing domain</scene> of LARS is responsible for hydrolysing mischarged tRNA, such as ile-tRNA<sup>leu</sup>, and releasing it for a subsequent round of aminoacylation. This editing function allows cells to achieve a tRNA charging error rate of less than 1:10,000<ref>doi: 10.1016/j.jmb.2009.04.073</ref>. The domain itself is an β sandwich connected to the catalytic domain by a flexible loop that allows a hinge action to bind aminoacylated tRNAs for error checking. | ||
| + | |||
| + | [[Image:Editing.JPG]] | ||
The binding pocket of the editing domain acts as a reciprocal sieve to the catalytic domain. The catalytic domain cannot distinguish between amino acids that are similar to, or smaller than leucine. The binding pocket of the editing site can accommodate these similar or smaller amino acids but excludes leucine due to a steric clash with a highly conserved theronine<ref>doi: 10.1016/j.jmb.2009.04.073</ref>. Together, the catalytic site discriminates against larger or hydrophobically different amino acids and the editing site hydrolyzes smaller, structurally similar amino acids producing a very low error rate<ref>DOI: 10.1042/BJ20051249</ref>. | The binding pocket of the editing domain acts as a reciprocal sieve to the catalytic domain. The catalytic domain cannot distinguish between amino acids that are similar to, or smaller than leucine. The binding pocket of the editing site can accommodate these similar or smaller amino acids but excludes leucine due to a steric clash with a highly conserved theronine<ref>doi: 10.1016/j.jmb.2009.04.073</ref>. Together, the catalytic site discriminates against larger or hydrophobically different amino acids and the editing site hydrolyzes smaller, structurally similar amino acids producing a very low error rate<ref>DOI: 10.1042/BJ20051249</ref>. | ||
Revision as of 13:47, 3 May 2018
| |||||||||||
3D Structure of LARS
Updated on 03-May-2018
References
- ↑ Mirande M. The Aminoacyl-tRNA Synthetase Complex. Subcell Biochem. 2017;83:505-522. doi: 10.1007/978-3-319-46503-6_18. PMID:28271488 doi:http://dx.doi.org/10.1007/978-3-319-46503-6_18
- ↑ Han JM, Kim JY, Kim S. Molecular network and functional implications of macromolecular tRNA synthetase complex. Biochem Biophys Res Commun. 2003 Apr 18;303(4):985-93. doi: , 10.1016/s0006-291x(03)00485-6. PMID:12684031 doi:http://dx.doi.org/10.1016/s0006-291x(03)00485-6
- ↑ Raina M, Elgamal S, Santangelo TJ, Ibba M. Association of a multi-synthetase complex with translating ribosomes in the archaeon Thermococcus kodakarensis. FEBS Lett. 2012 Jul 30;586(16):2232-8. doi: 10.1016/j.febslet.2012.05.039. Epub, 2012 Jun 7. PMID:22683511 doi:http://dx.doi.org/10.1016/j.febslet.2012.05.039
- ↑ Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012 Apr 13;149(2):410-24. doi: 10.1016/j.cell.2012.02.044. Epub 2012 Mar, 15. PMID:22424946 doi:http://dx.doi.org/10.1016/j.cell.2012.02.044
- ↑ Seiradake E, Mao W, Hernandez V, Baker SJ, Plattner JJ, Alley MR, Cusack S. Crystal structures of the human and fungal cytosolic Leucyl-tRNA synthetase editing domains: A structural basis for the rational design of antifungal benzoxaboroles. J Mol Biol. 2009 Jul 10;390(2):196-207. Epub 2009 May 6. PMID:19426743 doi:10.1016/j.jmb.2009.04.073
- ↑ Palencia A, Crepin T, Vu MT, Lincecum TL Jr, Martinis SA, Cusack S. Structural dynamics of the aminoacylation and proofreading functional cycle of bacterial leucyl-tRNA synthetase. Nat Struct Mol Biol. 2012 Jun 10. doi: 10.1038/nsmb.2317. PMID:22683997 doi:10.1038/nsmb.2317
- ↑ Cusack S, Yaremchuk A, Tukalo M. The 2 A crystal structure of leucyl-tRNA synthetase and its complex with a leucyl-adenylate analogue. EMBO J. 2000 May 15;19(10):2351-61. PMID:10811626 doi:10.1093/emboj/19.10.2351
- ↑ doi: https://dx.doi.org/10.1016/S1097-2765(03)00098-4
- ↑ Seiradake E, Mao W, Hernandez V, Baker SJ, Plattner JJ, Alley MR, Cusack S. Crystal structures of the human and fungal cytosolic Leucyl-tRNA synthetase editing domains: A structural basis for the rational design of antifungal benzoxaboroles. J Mol Biol. 2009 Jul 10;390(2):196-207. Epub 2009 May 6. PMID:19426743 doi:10.1016/j.jmb.2009.04.073
- ↑ Seiradake E, Mao W, Hernandez V, Baker SJ, Plattner JJ, Alley MR, Cusack S. Crystal structures of the human and fungal cytosolic Leucyl-tRNA synthetase editing domains: A structural basis for the rational design of antifungal benzoxaboroles. J Mol Biol. 2009 Jul 10;390(2):196-207. Epub 2009 May 6. PMID:19426743 doi:10.1016/j.jmb.2009.04.073
- ↑ Liu Y, Liao J, Zhu B, Wang ED, Ding J. Crystal structures of the editing domain of Escherichia coli leucyl-tRNA synthetase and its complexes with Met and Ile reveal a lock-and-key mechanism for amino acid discrimination. Biochem J. 2006 Mar 1;394(Pt 2):399-407. PMID:16277600 doi:10.1042/BJ20051249
- ↑ Palencia A, Crepin T, Vu MT, Lincecum TL Jr, Martinis SA, Cusack S. Structural dynamics of the aminoacylation and proofreading functional cycle of bacterial leucyl-tRNA synthetase. Nat Struct Mol Biol. 2012 Jun 10. doi: 10.1038/nsmb.2317. PMID:22683997 doi:10.1038/nsmb.2317
- ↑ Palencia A, Crepin T, Vu MT, Lincecum TL Jr, Martinis SA, Cusack S. Structural dynamics of the aminoacylation and proofreading functional cycle of bacterial leucyl-tRNA synthetase. Nat Struct Mol Biol. 2012 Jun 10. doi: 10.1038/nsmb.2317. PMID:22683997 doi:10.1038/nsmb.2317
- ↑ Palencia A, Crepin T, Vu MT, Lincecum TL Jr, Martinis SA, Cusack S. Structural dynamics of the aminoacylation and proofreading functional cycle of bacterial leucyl-tRNA synthetase. Nat Struct Mol Biol. 2012 Jun 10. doi: 10.1038/nsmb.2317. PMID:22683997 doi:10.1038/nsmb.2317
- ↑ Cusack S, Yaremchuk A, Tukalo M. The 2 A crystal structure of leucyl-tRNA synthetase and its complex with a leucyl-adenylate analogue. EMBO J. 2000 May 15;19(10):2351-61. PMID:10811626 doi:10.1093/emboj/19.10.2351
- ↑ Cusack S, Yaremchuk A, Tukalo M. The 2 A crystal structure of leucyl-tRNA synthetase and its complex with a leucyl-adenylate analogue. EMBO J. 2000 May 15;19(10):2351-61. PMID:10811626 doi:10.1093/emboj/19.10.2351
- ↑ Palencia A, Crepin T, Vu MT, Lincecum TL Jr, Martinis SA, Cusack S. Structural dynamics of the aminoacylation and proofreading functional cycle of bacterial leucyl-tRNA synthetase. Nat Struct Mol Biol. 2012 Jun 10. doi: 10.1038/nsmb.2317. PMID:22683997 doi:10.1038/nsmb.2317