We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Jennifer Taylor/Sandbox 3
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
==Introduction== | ==Introduction== | ||
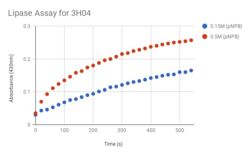
| - | Beginning in 2000, the National Institutes of Health’s Protein Structure Initiative was a fifteen-year program that worked to solve the structures of many proteins with known DNA sequences. The present study analyzes one such protein, known as 3H04, a predicted alpha/beta hydrolase found in Escherichia Coli. The specific objective of this study is to functionally characterize 3H04 based on structural analysis of the protein. In silico enzyme characterization for 3H04 was carried out using BLAST, Pfam, and Dali servers, followed by active site analysis using the PyMOL plug-in ProMOL. This structural analysis suggests PDB entries 1AZW and 1YSC - a triacylglycerol lipase, respectively - are most similar to 3H04; however 1TAH and 3H04 share the most catalytic triad similarity. To investigate the lipase activity of 3H04 in vitro, recombinant his-tagged protein was isolated from E. coli. | + | Beginning in 2000, the National Institutes of Health’s Protein Structure Initiative was a fifteen-year program that worked to solve the structures of many proteins with known DNA sequences. The present study analyzes one such protein, known as 3H04, a predicted alpha/beta hydrolase found in Escherichia Coli. The specific objective of this study is to functionally characterize 3H04 based on structural analysis of the protein. ''In silico'' enzyme characterization for 3H04 was carried out using BLAST, Pfam, and Dali servers, followed by active site analysis using the PyMOL plug-in ProMOL. This structural and protein sequence analysis suggests PDB entries 1AZW and 1YSC - a triacylglycerol lipase, respectively - are most similar to 3H04; however 1TAH and 3H04 share the most catalytic triad similarity. To investigate the lipase activity of 3H04 ''in vitro'', recombinant his-tagged protein was isolated from E. coli. |
| + | |||
| + | Proteins are macromolecules that are present in nearly every earthly organism and represent a vast array of functions. Scientists have been able to structurally solve proteins at a faster rate than they have been able to identify the functions of these same proteins. In an effort to close this gap, the National Institutes of Health launched the Protein Structure Initiative, which was a fifteen-year program, starting in 2000, that worked to solve the structures of many proteins with known DNA sequences. However, there are still hundreds proteins housed within the Protein Data Bank that have unidentified functions. Researchers are continuing to advocate for further analysis of these proteins through the development of BASIL modules that explain how to use ProMOL to analyzes the structural similarities between the active sites of proteins. | ||
| - | Proteins are macromolecules that are present in nearly every earthly organism and represent a vast array of functions. Studies of these polypeptides often lead to greater understanding of molecular pathways that may result in advantageous therapies. Scientists have been able to structurally solve proteins at a faster rate than they have been able to identify the functions of these same proteins. In an effort to close this gap, the National Institutes of Health launched the Protein Structure Initiative, which was a fifteen-year program, starting in 2000, that worked to solve the structures of many proteins with known DNA sequences. However, there are still hundreds proteins housed within the Protein Data Bank that have unidentified functions. Researchers are continuing to advocate for further analysis of these proteins through the development of BASIL modules that explain how to use ProMOL to analyzes the structural similarities between the active sites of proteins. | ||
== Overview == | == Overview == | ||
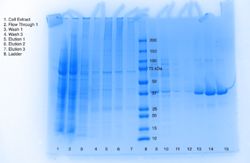
This project seeks to determine the function of a protein with an identified structure. The query protein, in this case, can be identified by the Protein Data Bank Identification (PDB ID) code 3H04. 3H04 is a alpha/beta hydrolase found in Escherichia Coli, a bacterium that resides in the lower intestines of warm-blooded mammals. The more specific purpose of this study is to characterize the function of 3H04. Plasmid purification is just one method for identifying an unidentified protein. Protein purification in bacterial cells requires the plasmids, a small circular double-stranded DNA molecule, to be purified and identified. The purification of the plasmid is meant to isolate and purify the plasmid DNA. The protein in the plasmid is identified through bacterial cells, and grows against ampicillin so the bacterial cells are growing against the antibiotic; the cells that grow have AmpR (Ampicillin Resistance gene). And in efforts to characterize our protein, 3H04, we are comparing it to other proteins with significant parts in common to hopefully help characterize the function of 3H04. The comparison of the different aspects of our protein to different aspects of other proteins also helped us determine our assay. | This project seeks to determine the function of a protein with an identified structure. The query protein, in this case, can be identified by the Protein Data Bank Identification (PDB ID) code 3H04. 3H04 is a alpha/beta hydrolase found in Escherichia Coli, a bacterium that resides in the lower intestines of warm-blooded mammals. The more specific purpose of this study is to characterize the function of 3H04. Plasmid purification is just one method for identifying an unidentified protein. Protein purification in bacterial cells requires the plasmids, a small circular double-stranded DNA molecule, to be purified and identified. The purification of the plasmid is meant to isolate and purify the plasmid DNA. The protein in the plasmid is identified through bacterial cells, and grows against ampicillin so the bacterial cells are growing against the antibiotic; the cells that grow have AmpR (Ampicillin Resistance gene). And in efforts to characterize our protein, 3H04, we are comparing it to other proteins with significant parts in common to hopefully help characterize the function of 3H04. The comparison of the different aspects of our protein to different aspects of other proteins also helped us determine our assay. | ||
Revision as of 18:06, 20 May 2018
3H04 Test Page
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644