AppA protein BLUF domain
From Proteopedia
(Difference between revisions)
| Line 17: | Line 17: | ||
==Further Analyses== | ==Further Analyses== | ||
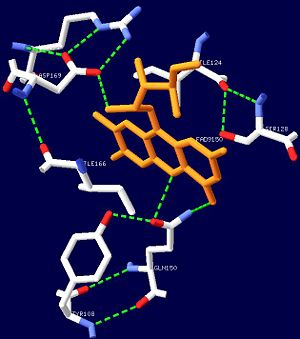
| - | While | + | While Asn132, Gln150, As169, Arg171, His172 and Ser110 are completely conserved residues, Asn131, Arg165 and Ser128 are only moderately conserved. This network surrounding FAD is thought to be highly conserved in all BLUF domains. In order to study the importance of the interactions between the isoalloxaizine ring of FAD, and Asn131, Asn132 and Gln150, the amino acids were replaced with alanine residues<ref name="one" />. It was observed that the spectra of the mutants were qualitatively similar to that of the wild-type. However, the low energy absorbance peak of N132A (Asn132 replaced with Ala) was blue shifted compared to the wildtype by 6nm<ref name="one" />. This blue shift can be accounted for by the lack of hydrogen bonding between the side-chain and isoalloxazine ring<ref name="one" />. In the wild-type protein, Asn132 forms 2 hydrogen bonds with FAD; the absence of these bonds allows relocation of an electron on FAD, resulting on the spectral blue shift. |
| - | The | + | The Q140A mutant showed very little spectral changes during illumination. These results suggest that Gln150 is critical for light reaction in BLUF domains and is thus totally conserved<ref name="one" />. Transient bleaching of flavin was observed instead of red-shifting; this suggests that light excitation of flavin in Q150A resulted in photoreduction of flavin<ref name="one" />. In general, a free flavin in solution is sequentially reduced with two electrons upon exposure to light. The first electron reduces the double bond at N5 and the second electron reduces at N1 of the isoalloxazine ring. In BLUF proteins, the role of Gln50 may be to prevent the initial photoreduction at N5 and allow a reaction leading to the spectral shift to a longer wavelength. This may be achieved by the hydrogen bonding between the amide N of Gln50 and the N5 of the isoalloxazine ring of FAD. |
Similar hydrogen bonding networks surrounding the isoalloxazine ring are found in other light sensing proteins such as the LOV proteins<ref name="one" />. The most critical residue in the flavin sensor proteins appears to be the one that interacts closely with N5 of the isoalloxazine ring<ref name="one" />. | Similar hydrogen bonding networks surrounding the isoalloxazine ring are found in other light sensing proteins such as the LOV proteins<ref name="one" />. The most critical residue in the flavin sensor proteins appears to be the one that interacts closely with N5 of the isoalloxazine ring<ref name="one" />. | ||
Revision as of 13:31, 24 May 2018
| |||||||||||
3D structures of BLUF domain protein
Updated on 24-May-2018
2byc - RsBLUF dark structure - Rhodobacter sphaeroides
2iyg, 2iyi - RsBLUF dark structure BLUF domain (mutant)
1x0p - BLUF - Thermosynechococcus elongatus
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 Kita A, Okajima K, Morimoto Y, Ikeuchi M, Miki K. Structure of a cyanobacterial BLUF protein, Tll0078, containing a novel FAD-binding blue light sensor domain. J Mol Biol. 2005 May 27;349(1):1-9. Epub 2005 Apr 9. PMID:15876364 doi:10.1016/j.jmb.2005.03.067
- ↑ van der Horst MA, Hellingwerf KJ. Photoreceptor proteins, "star actors of modern times": a review of the functional dynamics in the structure of representative members of six different photoreceptor families. Acc Chem Res. 2004 Jan;37(1):13-20. PMID:14730990 doi:10.1021/ar020219d
- ↑ 3.0 3.1 Masuda S, Bauer CE. AppA is a blue light photoreceptor that antirepresses photosynthesis gene expression in Rhodobacter sphaeroides. Cell. 2002 Sep 6;110(5):613-23. PMID:12230978
- ↑ Laan W, van der Horst MA, van Stokkum IH, Hellingwerf KJ. Initial characterization of the primary photochemistry of AppA, a blue-light-using flavin adenine dinucleotide-domain containing transcriptional antirepressor protein from Rhodobacter sphaeroides: a key role for reversible intramolecular proton transfer from the flavin adenine dinucleotide chromophore to a conserved tyrosine? Photochem Photobiol. 2003 Sep;78(3):290-7. PMID:14556317
- ↑ Hasegawa K, Masuda S, Ono TA. Spectroscopic analysis of the dark relaxation process of a photocycle in a sensor of blue light using FAD (BLUF) protein Slr1694 of the cyanobacterium Synechocystis sp. PCC6803. Plant Cell Physiol. 2005 Jan;46(1):136-46. Epub 2005 Jan 19. PMID:15659451 doi:10.1093/pcp/pci003
- ↑ 6.0 6.1 Iseki M, Matsunaga S, Murakami A, Ohno K, Shiga K, Yoshida K, Sugai M, Takahashi T, Hori T, Watanabe M. A blue-light-activated adenylyl cyclase mediates photoavoidance in Euglena gracilis. Nature. 2002 Feb 28;415(6875):1047-51. PMID:11875575 doi:10.1038/4151047a
- ↑ Yuan H, Anderson S, Masuda S, Dragnea V, Moffat K, Bauer C. Crystal structures of the Synechocystis photoreceptor Slr1694 reveal distinct structural states related to signaling. Biochemistry. 2006 Oct 24;45(42):12687-94. PMID:17042486 doi:10.1021/bi061435n
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Amanda Cookhouse, Jaime Prilusky