GABA receptor
From Proteopedia
| Line 3: | Line 3: | ||
== Introduction == | == Introduction == | ||
| - | '''GABA''' (i.e. gamma-aminobutyric acid) is the primary inhibitory neurotransmitter of the vertebrate central nervous system (Kerr, 1995). GABA can bind one of two different receptor proteins, each using a discrete mechanism to elicit a cellular response. Upon binding with GABA, GABAB receptors utilize a second messenger amplification pathway that ultimately results in an inhibitory signal for neuronal transmission. This pathway for signal transmission differs from GABAA receptors, which are considered ligand-gated ion channels as the binding of GABA results in the opening of ion channels leading to the inhibition of a neuronal signal. | + | '''GABA''' (i.e. gamma-aminobutyric acid) is the primary inhibitory neurotransmitter of the vertebrate central nervous system (Kerr, 1995). GABA can bind one of two different receptor proteins, each using a discrete mechanism to elicit a cellular response. Upon binding with GABA, '''GABAB receptors''' utilize a second messenger amplification pathway that ultimately results in an inhibitory signal for neuronal transmission. This pathway for signal transmission differs from '''GABAA receptors''', which are considered ligand-gated ion channels as the binding of GABA results in the opening of ion channels leading to the inhibition of a neuronal signal. |
== Structure == | == Structure == | ||
| Line 19: | Line 19: | ||
A possible therapeutic approach utilizing GABAB receptors would be for the treatment of substance use disorder (i.e. drug addiction). The GABAB receptor has been found to play a crucial role in mediating behavioral and molecular effects of drug abuse and could be used as a potential anti-addictive therapeutic strategy (Filip, 2015). Agonists of GABAB receptors can promote abstinence or decrease and control the reinforcing effects of drugs on the mind (Kerr 2005). | A possible therapeutic approach utilizing GABAB receptors would be for the treatment of substance use disorder (i.e. drug addiction). The GABAB receptor has been found to play a crucial role in mediating behavioral and molecular effects of drug abuse and could be used as a potential anti-addictive therapeutic strategy (Filip, 2015). Agonists of GABAB receptors can promote abstinence or decrease and control the reinforcing effects of drugs on the mind (Kerr 2005). | ||
| - | + | ||
| + | == 3D structures of GABA receptor == | ||
| + | [[GABA receptor 3D structures]] | ||
| + | |||
</StructureSection> | </StructureSection> | ||
== 3D structures of GABA receptor == | == 3D structures of GABA receptor == | ||
| Line 28: | Line 31: | ||
**[[4cof]] – hGABAA subunit β-3 - human<br /> | **[[4cof]] – hGABAA subunit β-3 - human<br /> | ||
| + | **[[6i53]], [[6hup]], [[6huo]], [[6huk]], [[6huj]], [[6hug]] – hGABAA subunits β-3 + α-1+γ-2 + megabody – Cryo EM<br /> | ||
| + | **[[6d6u]], [[6d6t]] – hGABAA subunits β-3 +α-1+γ-2 + antibody + GABA + flumazenil – Cryo EM<br /> | ||
| + | **[[6a96]] – hGABAA subunits β-3 +α-5 + nanobody + GABA – Cryo EM<br /> | ||
**[[4u90]], [[4tk4]], [[4tk3]], [[4tk2]], [[4tk1]] – rGABAA subunit α-3 (mutant) + gephyrin - rat<br /> | **[[4u90]], [[4tk4]], [[4tk3]], [[4tk2]], [[4tk1]] – rGABAA subunit α-3 (mutant) + gephyrin - rat<br /> | ||
| + | **[[6dw1]], [[6dw0]] – rGABAA subunits β-1 +α-1+γ-2 + GABA – Cryo EM<br /> | ||
* GABA-B receptor | * GABA-B receptor | ||
| - | **[[ | + | **[[6hkc]] – hGABAB subunit 1 sushi 1 - NMR<br /> |
**[[4f11]], [[4f12]] – hGABAB subunit 2 extracellular domain <br /> | **[[4f11]], [[4f12]] – hGABAB subunit 2 extracellular domain <br /> | ||
**[[4mqe]] – hGABAB subunit 1,2 extracellular domains <br /> | **[[4mqe]] – hGABAB subunit 1,2 extracellular domains <br /> | ||
**[[4mqf]], [[4mr7]], [[4mr8]], [[4mr9]], [[4mrm]], [[4ms1]], [[4ms3]], [[4ms4]] – hGABAB subunit 1,2 extracellular domains + antagonist<br /> | **[[4mqf]], [[4mr7]], [[4mr8]], [[4mr9]], [[4mrm]], [[4ms1]], [[4ms3]], [[4ms4]] – hGABAB subunit 1,2 extracellular domains + antagonist<br /> | ||
**[[4pas]] – hGABAB subunit 1,2 coiled-coil domains <br /> | **[[4pas]] – hGABAB subunit 1,2 coiled-coil domains <br /> | ||
| + | **[[1srz]], [[1ss2]] – rGABAB subunit 1 sushi 2 - NMR<br /> | ||
**[[5gwm]] – DmGABAB subtype 1+ subtype 3 coiled-coil domains – ''Drosophila melanogaster''<br /> | **[[5gwm]] – DmGABAB subtype 1+ subtype 3 coiled-coil domains – ''Drosophila melanogaster''<br /> | ||
**[[5x9x]] – DmGABAB subtype 1+ subtype 2 coiled-coil domains <br /> | **[[5x9x]] – DmGABAB subtype 1+ subtype 2 coiled-coil domains <br /> | ||
Revision as of 09:59, 4 July 2019
| |||||||||||
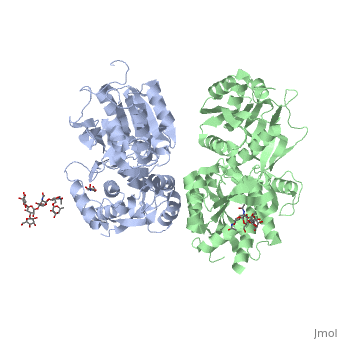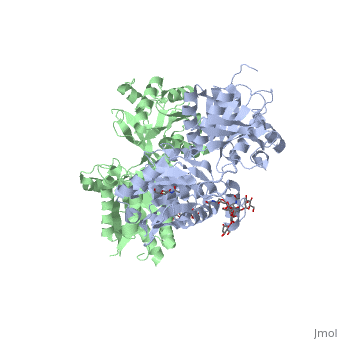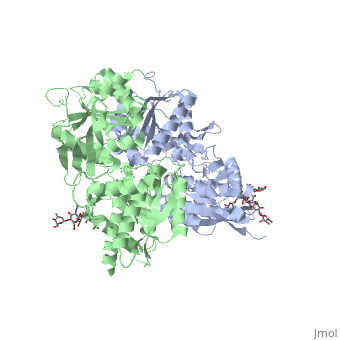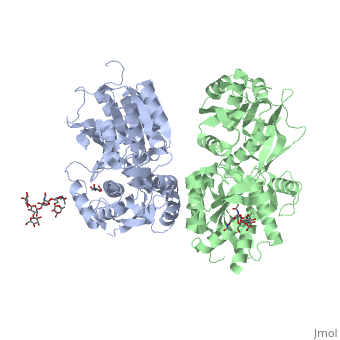
3D structures of GABA receptor
Updated on 04-July-2019
References
Bettler, B., Kaupmann, K., Mosbacher, J., & Gassmann, M. (2004). Molecular structure and physiological functions of GABAB receptors. Physiological reviews, 84(3), 835-867.
Citrome, L., Javitt, D., Kantrowitz, J. (2009). GABAB Receptors, Schizophrenia and Sleep Dysfunction. CNS Drugs, 23(8), 681-691.
Cryan, J.F., Kaupman, K. (2005). Don’t worry ‘B’ happy!: a role for GABAB receptors in anxiety and depression. Trends in Pharmacological Sciences, 26(1), 36-43.
Filip, M., Frankowska, M., et al., (2015). GABAB receptors as a therapuetic strategy in substance use disorders: Focus on positive allosteric modulators. Neuropharmacology. (38), 36-47.
Geng, Y., Bush, M., Mosyak, L., Wang, F., & Fan, Q. R. (2013). Structural mechanism of ligand activation in human GABAB receptor. Nature, 504(7479), 254-259.
Gumerov, V., Hegyi, H. (2015). MicroRNA-derived network analysis of differentially methylated genes in schizophrenia, implicating GABA receptor B1 [GABBR1] and protein kinase B [AKT1].Gumerov and Heygl Biology Direct. 10:59, 1-15.
Kerr, D. I. B., and J. Ong. "Clinical Potential of GABA B Receptor Modulators." CNS Drug Reviews. 11.3 (2005): 317-334.
Kerr, D. I. B., and J. Ong. "Gaba B receptors." Pharmacology & therapeutics. 67.2 (1995): 187-246.
Proteopedia Page Contributors and Editors (what is this?)
Jonathan Hurst, Gregory Holley, Alexander Berchansky, Michal Harel, Patrick Farrell, Joel L. Sussman, Karli Ribsam, Jaime Prilusky