User:Luis Netto/Sandbox 1
From Proteopedia
| Line 1: | Line 1: | ||
==Ohr == | ==Ohr == | ||
| - | <StructureSection load='1ZB8' size='340' side='right' caption='Caption | + | <StructureSection load='1ZB8' size='340' side='right' caption='Caption overall Ohr structure' scene=''> |
Ohr (Organic Hydroperoxide Resistance Protein)[[1zb8]] is a Cys based peroxidase <ref>PMID: 12540833</ref>,<ref>PMID: 12485986</ref> | Ohr (Organic Hydroperoxide Resistance Protein)[[1zb8]] is a Cys based peroxidase <ref>PMID: 12540833</ref>,<ref>PMID: 12485986</ref> | ||
that display higher preference for hydrogen peroxide (H2O2) than for organic hydroperoxides. Accordingly, bacteria with the gene for Ohr deleted displayed increased sensitivity for organic hydroperoxides but not for hydrogen peroxide <ref>PMID: 9573147</ref>. | that display higher preference for hydrogen peroxide (H2O2) than for organic hydroperoxides. Accordingly, bacteria with the gene for Ohr deleted displayed increased sensitivity for organic hydroperoxides but not for hydrogen peroxide <ref>PMID: 9573147</ref>. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | Ohr is a tight homo-dimer a [[Image:Ohr_black_white.png | thumb]] | ||
| - | |||
| - | |||
| Line 54: | Line 43: | ||
== Structural highlights == | == Structural highlights == | ||
| + | |||
| + | Ohr is a homo-dimer, with a symmetrical,oval shape. The two monomers are tightly wrapped around each other in a head-to-tail orientation to generate a compact quaternary structure.[[Image:Homodimer_Ohr.png]] | ||
Arg19 and Glu 50 of one chain (lets say chain A) compose the <scene name='78/785321/Catalytic_triad/1'>catalytic triad</scene> triad with Cys61 of the other chain (in this case chain B). | Arg19 and Glu 50 of one chain (lets say chain A) compose the <scene name='78/785321/Catalytic_triad/1'>catalytic triad</scene> triad with Cys61 of the other chain (in this case chain B). | ||
| + | Ohr is a tight homo-dimer a | ||
| - | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 21:06, 31 May 2018
Contents |
Ohr
| |||||||||||
It is involved in the response of
Disease
related with relevance
Relevance
Oxidants such as fatty acid hydroperoxides are signaling molecules involved in host-pathogen interactions, and therefore, their levels are strictly controlled by peroxidases and other mechanisms [1±4]. Ohr (Organic hydroperoxide resistance) proteins are Cys-based, dithiol-dependent peroxidases that display unique biochemical and structural properties [5,6]. Ohr enzymes play central roles in the bacterial response to peroxynitrite and fatty acid hydroperoxides, two oxidants involved in host±pathogen interactions [1]. These enzymes are found in bacteria and fungi, and they are absent in their hosts (plants and animals) [7], making them promising targets for drug discovery. Some examples of pathogenic bacteria that express Ohr proteins are Pseudomonas aeruginosa, Vibrio cholerae and Xyllela fastidiosa [7]. Xylella fastidiosa is a plant pathogen with agronomic interest, causing disease in citrus, grapes and olives [8].
Structural highlights
Ohr is a homo-dimer, with a symmetrical,oval shape. The two monomers are tightly wrapped around each other in a head-to-tail orientation to generate a compact quaternary structure.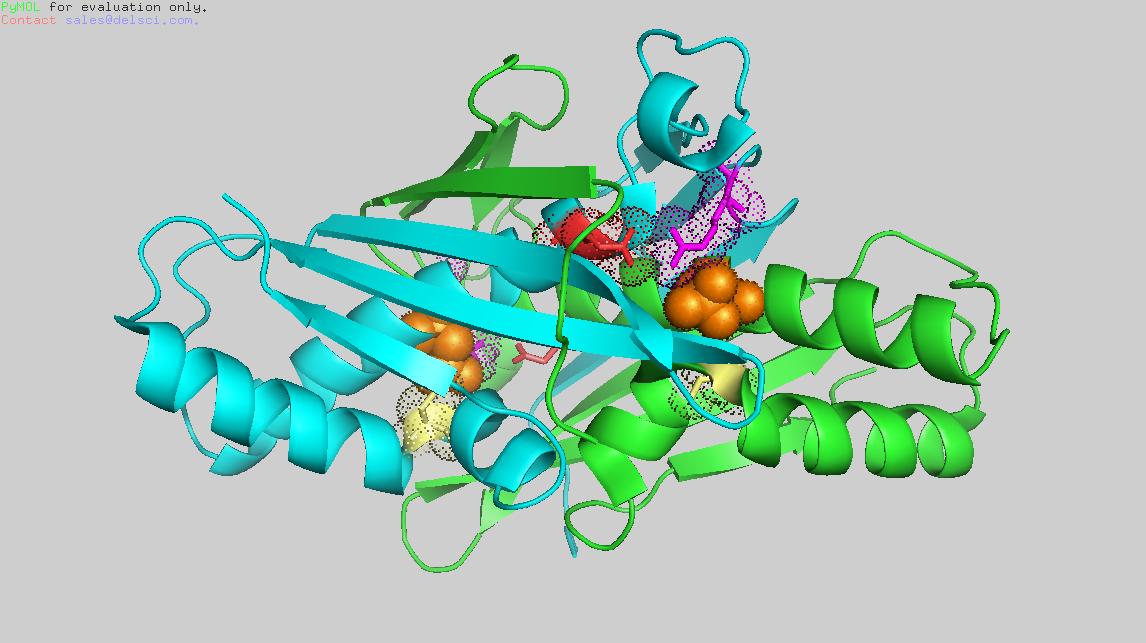
Arg19 and Glu 50 of one chain (lets say chain A) compose the triad with Cys61 of the other chain (in this case chain B).
Ohr is a tight homo-dimer a
References
- ↑ Cussiol JR, Alves SV, de Oliveira MA, Netto LE. Organic hydroperoxide resistance gene encodes a thiol-dependent peroxidase. J Biol Chem. 2003 Mar 28;278(13):11570-8. doi: 10.1074/jbc.M300252200. Epub 2003 , Jan 22. PMID:12540833 doi:http://dx.doi.org/10.1074/jbc.M300252200
- ↑ Lesniak J, Barton WA, Nikolov DB. Structural and functional characterization of the Pseudomonas hydroperoxide resistance protein Ohr. EMBO J. 2002 Dec 16;21(24):6649-59. PMID:12485986
- ↑ Mongkolsuk S, Praituan W, Loprasert S, Fuangthong M, Chamnongpol S. Identification and characterization of a new organic hydroperoxide resistance (ohr) gene with a novel pattern of oxidative stress regulation from Xanthomonas campestris pv. phaseoli. J Bacteriol. 1998 May;180(10):2636-43. PMID:9573147
