Green Fluorescent Protein
From Proteopedia
| Line 11: | Line 11: | ||
Shimomura was originally looking only to isolate the blue luminescent protein of ''Aequorea victoria'', traditionally thought to be [[luciferase]], but it would soon become apparent that the glow was in fact due to aequorin, a substance related, but slightly varying from luciferase.<ref name="Haldar" /><ref name="Shimomura" /> However, the light emitted from aequorin still differed from the light emitted from the wild jellyfish. This quandary led to the discovery of the green fluorescent protein responsible for this disparity, but sufficient amounts of the protein could not be collected for study until 1979. The journey to discover the nature of GFP had begun.<ref name="Shimomura" /> | Shimomura was originally looking only to isolate the blue luminescent protein of ''Aequorea victoria'', traditionally thought to be [[luciferase]], but it would soon become apparent that the glow was in fact due to aequorin, a substance related, but slightly varying from luciferase.<ref name="Haldar" /><ref name="Shimomura" /> However, the light emitted from aequorin still differed from the light emitted from the wild jellyfish. This quandary led to the discovery of the green fluorescent protein responsible for this disparity, but sufficient amounts of the protein could not be collected for study until 1979. The journey to discover the nature of GFP had begun.<ref name="Shimomura" /> | ||
| - | In the 1990’s, Douglas Prasher, Frank | + | In the 1990’s, Douglas Prasher, Frank Prendergast, and co-workers successfully cloned the gene that encoded for GFP. Martin Chalfie further pursued this line of work and was eventually able to express GFP in heterologous systems such as E. coli and C. elegans. Chalfie’s research provided the first evidence that GFP was unique as it did not require the presence of any exogenous substance or cofactor for fluorescence.<ref name="Haldar" /> The lack for the need for a cofactor proved that the cloned GFP gene contained all the information necessary for posttranslational synthesis of the chromophore. <ref name="Tsien" /> |
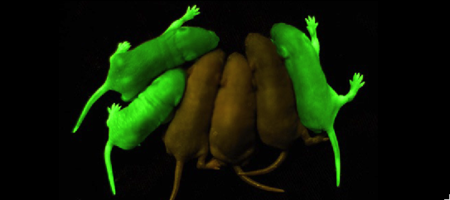
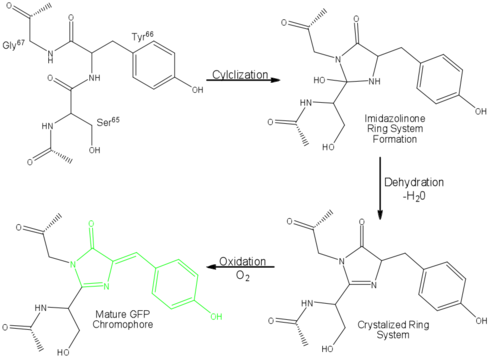
Roger Tsien and co-workers were intrigued by the absence of a necessary cofactor and began to research the structure of GFP and how it relates to its fluorescence. They discovered that a helix within the beta barrel structure of GFP actually contained a fluorophore responsible for fluorescence. In researching its structure, they were able to develop GFP derivatives with improved fluorescence and photo-stability. Shimomura, Chalfie, and Tsien were each recognized for their work involving GFP with the Nobel Prize in 2008.<ref name="Haldar" /> In the time since the work of these three researchers, GFP has been successfully expressed and utilized in bacteria, yeast, slime mold, plants, drosophila fruit flies, zebra-fish, and mammalian cells.<ref name="Yang" /> Below, mice have had GFP inserted into their genomes for studies in neurology. | Roger Tsien and co-workers were intrigued by the absence of a necessary cofactor and began to research the structure of GFP and how it relates to its fluorescence. They discovered that a helix within the beta barrel structure of GFP actually contained a fluorophore responsible for fluorescence. In researching its structure, they were able to develop GFP derivatives with improved fluorescence and photo-stability. Shimomura, Chalfie, and Tsien were each recognized for their work involving GFP with the Nobel Prize in 2008.<ref name="Haldar" /> In the time since the work of these three researchers, GFP has been successfully expressed and utilized in bacteria, yeast, slime mold, plants, drosophila fruit flies, zebra-fish, and mammalian cells.<ref name="Yang" /> Below, mice have had GFP inserted into their genomes for studies in neurology. | ||
Revision as of 15:04, 24 August 2018
| |||||||||||
Contents |
3D Printed Physical Model of Green Fluorescent Protein (GFP)
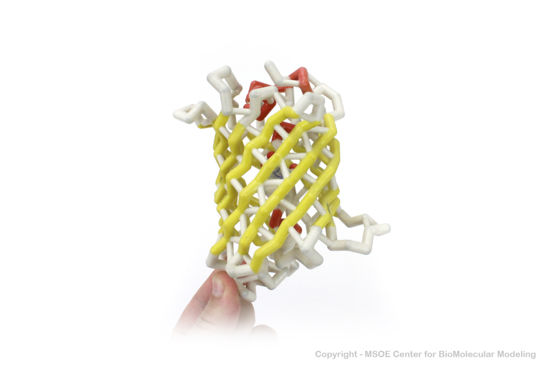
Shown below are 3D printed physical models of the Green Fluorescent Protein (GFP). The first alpha carbon backbone model is colored by three-strand repeats, including red, blue, purple, and yellow. The second alpha carbon backbone model is colored by secondary structure, with alpha helices red and beta sheets yellow. Both models show the fluorophore molecule at the center of the GFP structure.
The MSOE Center for BioMolecular Modeling
The MSOE Center for BioMolecular Modeling uses 3D printing technology to create physical models of protein and molecular structures, making the invisible molecular world more tangible and comprehensible. To view more protein structure models, visit our Model Gallery.
3D structures of Green Fluorescent Protein (Updated on 24-August-2018)
Reference for this Structure
Ormo M, Cubitt AB, Kallio K, Gross LA, Tsien RY, Remington SJ. 1996. Crystal structure of the Aequorea victoria green fluorescent protein. Science. 273(5280):1392-1395. DOI 10.1126/science.273.5280.1392.
References
- ↑ 1.0 1.1 [1], Protein Database (PDBsum): 1ema. European Bioinformatics (EBI); 2009.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 [2], Yang F, Moss LG, Phillips GN Jr. 1996. The molecular structure of green fluorescent protein. Biotechnology. 14: 1246-1251. DOI 10.1038/nbt1096-1246.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Tsien, Roger Y. 1998. The Green Fluorescent Protein. Annual Review in Biochemistry. 67:509-544.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 [3], Haldar S, Chattopadhyay A. 2009. The green journey. J Fluoresc. 19:1-2. DOI 10.1007/s10895-008-0455-6; biographical background on Douglas Prasher, Martin Chalfie and Roger Tsien.
- ↑ 5.0 5.1 5.2 5.3 [4], Shimomura O. The discovery of green fluorescent protein. Nobel Prize Lecture; 2009;; biographical background at Wikipedia.
- ↑ [5],Cowles D, Cowles J. Aequorea victoria. 2007. Walla Wall University.
- ↑ Primary structure at www.ebi.aci.uk.
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 8.11 8.12 8.13 Ormo M, Cubitt AB, Kallio K, Gross LA, Tsien RY, Remington SJ. 1996. Crystal structure of the Aequorea victoria green fluorescent protein. Science. 273(5280):1392-1395. DOI 10.1126/science.273.5280.1392.
- ↑ 9.00 9.01 9.02 9.03 9.04 9.05 9.06 9.07 9.08 9.09 9.10 9.11 9.12 9.13 Phillips GN Jr. Structure and dynamics of green fluorescent protein. Curr Opin Struct Biol. 1997 Dec;7(6):821-7. PMID:9434902
- ↑ Andrews BT, Gosavi S, Finke JM, Onuchic JN, Jennings PA. The dual-basin landscape in GFP folding. Proc Natl Acad Sci U S A. 2008 Aug 26;105(34):12283-8. Epub 2008 Aug 19. PMID:18713871
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 [6],Cubitt AB, Heim R, Adams SR, Boyd AE, Gross LA, Tsien R. 1995. Understanding, improving, and using green fluorescent protein. Trends in Biochemical Sciences. 20(11): 448-455. DOI 0.1016/S0968-0004(00)89099-4.
- ↑ Bublitz G, King BA, Boxer SG. 1998. Electronic structure of the chromophore in green fluorescent protein (GFP). Journal of the American Chemical Society. 120(36): 9370-9371. DOI 10.1021/ja98160e.
- ↑ van Thor JJ, Sage, JT. 2006. Charge transfer in green fluorescent protein. Photochemical & Photobiological Sciences. 5:597-602. DOI 10.1039/b516525c.
- ↑ 14.0 14.1 Lammich L, Petersen MA, Nielsen MB, Andersen LH. The gas-phase absorption spectrum of a neutral GFP model chromophore. Biophys J. 2007 Jan 1;92(1):201-7. Epub 2006 Oct 13. PMID:17040991 doi:10.1529/biophysj.106.093674
- ↑ Information about edge-face (CH/π) interactions.
- ↑ Fang C, Frontiera RR, Tran R, Mathies RA. Mapping GFP structure evolution during proton transfer with femtosecond Raman spectroscopy. Nature. 2009 Nov 12;462(7270):200-4. PMID:19907490 doi:10.1038/nature08527
Additional Resources
- For additional information, see: Colored & Bioluminescent Proteins
- First Glance
- PDBsum: 1ema
- RCSB PDB 1ema
- OCA
- UniProt: P42212
- Scop: P42212
- CATH: 1emaA00
- Pfam: PF01353
- InterPro: IPR000786
- GFP featured at the Molecule of the Month series of tutorials by David Goodsell.
- Inside green fluorescent protein - editor's summary that accompanied structural detail of GFP chromophore on the cover of Nature.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Wayne Decatur, Karsten Theis, Eran Hodis, Laura Carbone, Karl Oberholser, Mark Hoelzer, Joel L. Sussman, Alexander Berchansky, Jaime Prilusky, Marius Mihasan, Joseph M. Steinberger, David Canner