We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Journal:PLoS ONE:1
From Proteopedia
(Difference between revisions)

| Line 7: | Line 7: | ||
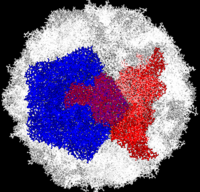
[[Image:Polio.png|left|200px|thumb|Complete Poliovirus 2 Virion (capsid) based on PDB entry [[1eah]], <font color='red'><b>example of 3-fold symmetry is in red</b></font>, <font color='blue'><b>example of 5-fold symmetry is in blue</b></font>]] | [[Image:Polio.png|left|200px|thumb|Complete Poliovirus 2 Virion (capsid) based on PDB entry [[1eah]], <font color='red'><b>example of 3-fold symmetry is in red</b></font>, <font color='blue'><b>example of 5-fold symmetry is in blue</b></font>]] | ||
| + | {{Clear}} | ||
Poliovirus is a member of the ''Picornaviridae''. Like other members of the ''Picornaviridae'', poliovirus RNA is encapsulated in an icosahedral structure (see the static image at the left or <scene name='Journal:TMP:1/Cv/2'>simplified model of capsid</scene>) formed from 60 <scene name='Journal:PLoS_ONE:1/Cv/2'>capsomeres</scene> with axes of <scene name='Journal:PLoS_ONE:1/Cv/3'>five-fold</scene> and <scene name='Journal:PLoS_ONE:1/Cv/5'>three-fold</scene> symmetry. So, <scene name='Journal:PLoS_ONE:1/Cv/7'>each capsomer participates</scene> in formation of <scene name='Journal:PLoS_ONE:1/Cv/9'>both kinds of symmetry</scene>. <scene name='Journal:PLoS_ONE:1/Cv/11'>A single capsomer contains one copy</scene> of viral capsid proteins <span style="color:lightskyblue;background-color:black;font-weight:bold;">VP1 (light blue)</span>, <span style="color:palegreen;background-color:black;font-weight:bold;">VP2 (pale green)</span>, <span style="color:sandybrown;background-color:black;font-weight:bold;">VP3 (light orange)</span> and <span style="color:magenta;background-color:black;font-weight:bold;">VP4 (light magenta)</span>. The binding site for the human poliovirus receptor is located <scene name='Journal:PLoS_ONE:1/Cv1/1'>in a canyon at the five-fold axis of symmetry (external viewpoint)</scene>. The VP1 of picornaviruses contain a <scene name='Journal:PLoS_ONE:1/Cv1/2'>hydrophobic pocket that is accessed through this canyon</scene>. This pocket is normally occupied by pocket factors, sphingosine-like molecules including palmitic and <scene name='Journal:PLoS_ONE:1/Cv1/3'>myristic acids </scene> and hydrophobic compounds, that stabilize the capsid and whose removal is a necessary prerequisite for uncoating. The <scene name='Journal:PLoS_ONE:1/Cv1/4'>broad-spectrum antiviral agent SCH48973 is observed binding in a pocket within the beta-barrel of VP1, in approximately the same location that natural pocket factors bind to polioviruses</scene> ([[1eah]]). SCH48973 forms predominantly hydrophobic interactions with the pocket residues. Flavanoids and flavonoids were shown to have antiviral activity ''in vitro'' against several picornaviruses including type 2 polioviruses. These compounds act primarily by occupying the hydrophobic pocket, thus interfering with virus uncoating. Compounds <scene name='Journal:PLoS_ONE:1/Iso/2'>3(2H)-Isoflavene (C2)</scene> and 6-chloro-3(2H)-Isoflavene (C10) are relatively similar to <scene name='Journal:PLoS_ONE:1/Sc/1'>SCH48973</scene> and exert their antiviral effect by interacting with amino acids in the viral capsid pockets. Ambiguous vaccine-derived poliovirus 2 (aVDPV) isolate <scene name='Journal:PLoS_ONE:1/F1/6'>SD-06-10</scene> was resistant to the antiviral effects of both C2 and C10 compounds. The ''in silico'' translated amino acid sequences of the capsid proteins of all of the aVDPV2 isolates and Sabin 2 (AY184220) were compared and amino acid substitutions in capsid proteins that were unique to SD-06-10 were determined. The positions of these unique substitutions were mapped onto the coordinates of type 2 poliovirus ([[1eah]]) using PyMOL and <scene name='Journal:PLoS_ONE:1/F1/5'>are represented by red spheres</scene>. <font color='blue'><b>The hydrophobic pocket is represented by blue spheres</b></font> and the position of amino-acid substitutions that were previously shown to result in loss of sensitivity <span style="color:yellow;background-color:black;font-weight:bold;">(yellow)</span> to or <span style="color:lime;background-color:black;font-weight:bold;">dependence</span> on isoflavenes are indicated by the yellow and green spheres, respectively. One unique substitution at <scene name='Journal:PLoS_ONE:1/F45/11'>amino acid Ser6 of VP3, was located in the central junction of the 5 capsomeres at the 5-fold axis</scene> of symmetry <scene name='Journal:PLoS_ONE:1/F45/12'>(click to enlarge)</scene> and another <scene name='Journal:PLoS_ONE:1/F43/4'>unique substitution at Ser206 of VP3, was located adjacent to the 3-fold axis of symmetry where they might influence capsid flexibility and or stability</scene>. | Poliovirus is a member of the ''Picornaviridae''. Like other members of the ''Picornaviridae'', poliovirus RNA is encapsulated in an icosahedral structure (see the static image at the left or <scene name='Journal:TMP:1/Cv/2'>simplified model of capsid</scene>) formed from 60 <scene name='Journal:PLoS_ONE:1/Cv/2'>capsomeres</scene> with axes of <scene name='Journal:PLoS_ONE:1/Cv/3'>five-fold</scene> and <scene name='Journal:PLoS_ONE:1/Cv/5'>three-fold</scene> symmetry. So, <scene name='Journal:PLoS_ONE:1/Cv/7'>each capsomer participates</scene> in formation of <scene name='Journal:PLoS_ONE:1/Cv/9'>both kinds of symmetry</scene>. <scene name='Journal:PLoS_ONE:1/Cv/11'>A single capsomer contains one copy</scene> of viral capsid proteins <span style="color:lightskyblue;background-color:black;font-weight:bold;">VP1 (light blue)</span>, <span style="color:palegreen;background-color:black;font-weight:bold;">VP2 (pale green)</span>, <span style="color:sandybrown;background-color:black;font-weight:bold;">VP3 (light orange)</span> and <span style="color:magenta;background-color:black;font-weight:bold;">VP4 (light magenta)</span>. The binding site for the human poliovirus receptor is located <scene name='Journal:PLoS_ONE:1/Cv1/1'>in a canyon at the five-fold axis of symmetry (external viewpoint)</scene>. The VP1 of picornaviruses contain a <scene name='Journal:PLoS_ONE:1/Cv1/2'>hydrophobic pocket that is accessed through this canyon</scene>. This pocket is normally occupied by pocket factors, sphingosine-like molecules including palmitic and <scene name='Journal:PLoS_ONE:1/Cv1/3'>myristic acids </scene> and hydrophobic compounds, that stabilize the capsid and whose removal is a necessary prerequisite for uncoating. The <scene name='Journal:PLoS_ONE:1/Cv1/4'>broad-spectrum antiviral agent SCH48973 is observed binding in a pocket within the beta-barrel of VP1, in approximately the same location that natural pocket factors bind to polioviruses</scene> ([[1eah]]). SCH48973 forms predominantly hydrophobic interactions with the pocket residues. Flavanoids and flavonoids were shown to have antiviral activity ''in vitro'' against several picornaviruses including type 2 polioviruses. These compounds act primarily by occupying the hydrophobic pocket, thus interfering with virus uncoating. Compounds <scene name='Journal:PLoS_ONE:1/Iso/2'>3(2H)-Isoflavene (C2)</scene> and 6-chloro-3(2H)-Isoflavene (C10) are relatively similar to <scene name='Journal:PLoS_ONE:1/Sc/1'>SCH48973</scene> and exert their antiviral effect by interacting with amino acids in the viral capsid pockets. Ambiguous vaccine-derived poliovirus 2 (aVDPV) isolate <scene name='Journal:PLoS_ONE:1/F1/6'>SD-06-10</scene> was resistant to the antiviral effects of both C2 and C10 compounds. The ''in silico'' translated amino acid sequences of the capsid proteins of all of the aVDPV2 isolates and Sabin 2 (AY184220) were compared and amino acid substitutions in capsid proteins that were unique to SD-06-10 were determined. The positions of these unique substitutions were mapped onto the coordinates of type 2 poliovirus ([[1eah]]) using PyMOL and <scene name='Journal:PLoS_ONE:1/F1/5'>are represented by red spheres</scene>. <font color='blue'><b>The hydrophobic pocket is represented by blue spheres</b></font> and the position of amino-acid substitutions that were previously shown to result in loss of sensitivity <span style="color:yellow;background-color:black;font-weight:bold;">(yellow)</span> to or <span style="color:lime;background-color:black;font-weight:bold;">dependence</span> on isoflavenes are indicated by the yellow and green spheres, respectively. One unique substitution at <scene name='Journal:PLoS_ONE:1/F45/11'>amino acid Ser6 of VP3, was located in the central junction of the 5 capsomeres at the 5-fold axis</scene> of symmetry <scene name='Journal:PLoS_ONE:1/F45/12'>(click to enlarge)</scene> and another <scene name='Journal:PLoS_ONE:1/F43/4'>unique substitution at Ser206 of VP3, was located adjacent to the 3-fold axis of symmetry where they might influence capsid flexibility and or stability</scene>. | ||
The positions of unique substitutions of SD-07-03 was also determined and were mapped onto the coordinates of type 2 poliovirus and <scene name='Journal:PLoS_ONE:1/F51/4'>are represented by red spheres</scene>. | The positions of unique substitutions of SD-07-03 was also determined and were mapped onto the coordinates of type 2 poliovirus and <scene name='Journal:PLoS_ONE:1/F51/4'>are represented by red spheres</scene>. | ||
Current revision
| |||||||||||
- ↑ Shulman LM, Sofer D, Manor Y, Mendelson E, Balanant J, Salvati AL, Delpeyroux F, Fiore L. Antiviral activity of 3(2H)- and 6-chloro-3(2H)-isoflavenes against highly diverged, neurovirulent vaccine-derived, type2 poliovirus sewage isolates. PLoS One. 2011;6(5):e18360. Epub 2011 May 25. PMID:21904594 doi:10.1371/journal.pone.0018360
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.