This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Green Fluorescent Protein
From Proteopedia
| Line 39: | Line 39: | ||
As the central α-helix is not located directly in the center of the β-barrel, cavities of differing area exist on either side of the chromophore. The larger cavity, consisting of about 135 Å,<ref name="Ormo" /> does not open out to the bulk solvent, but rather houses <scene name='Green_Fluorescent_Protein/Water_molecules/1'>four water molecules</scene>.<ref name="Ormo" /><ref name="Van">van Thor JJ, Sage, JT. 2006. Charge transfer in green fluorescent protein. Photochemical & Photobiological Sciences. 5:597-602. DOI 10.1039/b516525c.</ref> Had this space not been occupied, it would be expected to considerably destabilize the protein as a whole. The hydrogen bonding created by the presence of the water molecules, however, helps to link the buried <scene name='Green_Fluorescent_Protein/Gln69_glu222/1'>side chains</scene> of Glu<sup>222</sup> and Gln<sup>69</sup> that would otherwise be actively polar.<ref name="Ormo" /> Therefore, the water molecules are extremely important in establishing a hydrogen bonding network about the chromophor.<ref name="Lammich">PMID: 17040991</ref> | As the central α-helix is not located directly in the center of the β-barrel, cavities of differing area exist on either side of the chromophore. The larger cavity, consisting of about 135 Å,<ref name="Ormo" /> does not open out to the bulk solvent, but rather houses <scene name='Green_Fluorescent_Protein/Water_molecules/1'>four water molecules</scene>.<ref name="Ormo" /><ref name="Van">van Thor JJ, Sage, JT. 2006. Charge transfer in green fluorescent protein. Photochemical & Photobiological Sciences. 5:597-602. DOI 10.1039/b516525c.</ref> Had this space not been occupied, it would be expected to considerably destabilize the protein as a whole. The hydrogen bonding created by the presence of the water molecules, however, helps to link the buried <scene name='Green_Fluorescent_Protein/Gln69_glu222/1'>side chains</scene> of Glu<sup>222</sup> and Gln<sup>69</sup> that would otherwise be actively polar.<ref name="Ormo" /> Therefore, the water molecules are extremely important in establishing a hydrogen bonding network about the chromophor.<ref name="Lammich">PMID: 17040991</ref> | ||
| - | The opposite side of the chromophore, however, is within close proximity of several aromatic and polar side chains. Several <scene name='Green_Fluorescent_Protein/Polar_interactions/2'>polar interactions</scene> between the surrounding residues and the chromophore are present including: hydrogen bonds of His<sup>148</sup>, Thr<sup>203</sup>, and Ser<sup>205</sup> with the phenolic hydroxyl of Tyr<sup>66</sup>; Arg<sup>96</sup> and Gln<sup>94</sup> with the carbonyl of the imidazolidinone ring; and hydrogen bonds of Glu<sup>222</sup> with the side chain of Thr<sup>65</sup>. Additional hydrogen bonding in the area around the chromophore helps to stabilize Arg<sup>96</sup> in the protonated form, which suggests the presence of a partial negative charge on the carbonyl oxygen of the imidazolidinone ring in the deprotonated fluorophore.<ref name="Ormo" /> Arg<sup>96</sup> and Gln<sup>94</sup> in turn help to steady the imidazolidone.<ref name="Yang" /> Therefore, it is thought that Arg<sup>96</sup> is essential for the formation of the fluorophore by catalyzing the initial ring closure.<ref name="Ormo" /> Tyr<sup>145</sup> provides a stabilizing | + | The opposite side of the chromophore, however, is within close proximity of several aromatic and polar side chains. Several <scene name='Green_Fluorescent_Protein/Polar_interactions/2'>polar interactions</scene> between the surrounding residues and the chromophore are present including: hydrogen bonds of His<sup>148</sup>, Thr<sup>203</sup>, and Ser<sup>205</sup> with the phenolic hydroxyl of Tyr<sup>66</sup>; Arg<sup>96</sup> and Gln<sup>94</sup> with the carbonyl of the imidazolidinone ring; and hydrogen bonds of Glu<sup>222</sup> with the side chain of Thr<sup>65</sup>. Additional hydrogen bonding in the area around the chromophore helps to stabilize Arg<sup>96</sup> in the protonated form, which suggests the presence of a partial negative charge on the carbonyl oxygen of the imidazolidinone ring in the deprotonated fluorophore.<ref name="Ormo" /> Arg<sup>96</sup> and Gln<sup>94</sup> in turn help to steady the imidazolidone.<ref name="Yang" /> Therefore, it is thought that Arg<sup>96</sup> is essential for the formation of the fluorophore by catalyzing the initial ring closure.<ref name="Ormo" /> Tyr<sup>145</sup> provides a stabilizing <scene name='Green_Fluorescent_Protein/Edge_face_interaction/1'>edge-face interaction</scene><ref> [http://www.tim.hi-ho.ne.jp/dionisio/ Information about edge-face (CH/π) interactions].</ref> with the benzyl ring of the chromophore.<ref name="Ormo" /> The stability provided by the internal polar interactions are further augmented by the surrounding β-barrel. |
| - | <scene name='Green_Fluorescent_Protein/Edge_face_interaction/1'>edge-face interaction</scene><ref> [http://www.tim.hi-ho.ne.jp/dionisio/ Information about edge-face (CH/π) interactions].</ref> with the benzyl ring of the chromophore.<ref name="Ormo" /> The stability provided by the internal polar interactions are further augmented by the surrounding β-barrel. | + | |
The β-barrel provides a highly constrained environment that protects the chromophore from the bulk solvent,<ref name="Haldar" /> nearly creating the atmosphere of a vacuum.<ref name="Lammich" /> This is most likely responsible for the small [[Stoke’s shift]], or the small wavelength difference between excitation and emission.<ref name="Ormo" /> | The β-barrel provides a highly constrained environment that protects the chromophore from the bulk solvent,<ref name="Haldar" /> nearly creating the atmosphere of a vacuum.<ref name="Lammich" /> This is most likely responsible for the small [[Stoke’s shift]], or the small wavelength difference between excitation and emission.<ref name="Ormo" /> | ||
| Line 48: | Line 47: | ||
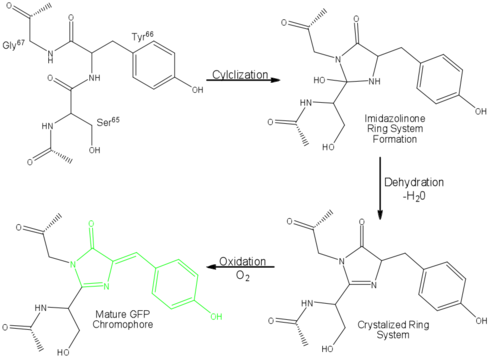
Findings show that fluorescence will not occur from a naked chromophore, but rather requires the protection of the β-can structure.<ref name="Cubitt" /> However, ''in crystallum'' GFP will exhibit a nearly identical fluorescence spectrum and lifetime when compared with aqueous GFP. These two elements point to a fluorescence that is not inherent to the isolated fluorophore,<ref name="Yang" /><ref name="Phillips" /> but rather from the auto-catalytic cyclization of the polypeptide sequence Ser<sup>65</sup>Tyr<sup>66</sup>Gly<sup>67</sup> and subsequent oxidation of Tyr<sup>66</sup>.<ref name="Phillips" /> However, this sequence is found in many proteins - why does GFP fluoresce? According to Phillips (1997), fluorophore formation is due to the close proximity of the backbone atoms between Ser<sup>65</sup>. and Gly<sup>67</sup> gained through a lack of sterical hindrance by the hydrogen atom side chain of glycine. In fact, no functional fluorescent proteins have been found in which any other amino acid other than glycine was found at position 67. Even so, there are still proteins that have this specific sequence, therefore, there must be another inherent property to GFP that is still left misunderstood.<ref name="Phillips" /> | Findings show that fluorescence will not occur from a naked chromophore, but rather requires the protection of the β-can structure.<ref name="Cubitt" /> However, ''in crystallum'' GFP will exhibit a nearly identical fluorescence spectrum and lifetime when compared with aqueous GFP. These two elements point to a fluorescence that is not inherent to the isolated fluorophore,<ref name="Yang" /><ref name="Phillips" /> but rather from the auto-catalytic cyclization of the polypeptide sequence Ser<sup>65</sup>Tyr<sup>66</sup>Gly<sup>67</sup> and subsequent oxidation of Tyr<sup>66</sup>.<ref name="Phillips" /> However, this sequence is found in many proteins - why does GFP fluoresce? According to Phillips (1997), fluorophore formation is due to the close proximity of the backbone atoms between Ser<sup>65</sup>. and Gly<sup>67</sup> gained through a lack of sterical hindrance by the hydrogen atom side chain of glycine. In fact, no functional fluorescent proteins have been found in which any other amino acid other than glycine was found at position 67. Even so, there are still proteins that have this specific sequence, therefore, there must be another inherent property to GFP that is still left misunderstood.<ref name="Phillips" /> | ||
| - | This quandary led Phillips to study the acid/base chemistry catalyzing the initial cyclization of the chromophore. He found that Arg<sup>96</sup> actually acts as a | + | This quandary led Phillips to study the acid/base chemistry catalyzing the initial cyclization of the chromophore. He found that Arg<sup>96</sup> actually acts as a <scene name='Green_Fluorescent_Protein/Arg96/1' >base</scene> by withdrawing electrons through hydrogen bonding with the carbonyl oxygen of Ser<sup>65</sup> to activate the carbonyl carbon for nucleophilic attack by the amide nitrogen of Gly<sup>67</sup>. This mechanism was further supported by ''ab initio'' calculations, as well as database searches of similar compounds and protein sequences. Through acid/base chemistry, the chromophore is stabilized by resonance.<ref name="Phillips" /> Femtosecond Raman spectroscopy has been used to map the alteration of the structure close the chromophore during excited-state protein transfer and shown that chromophore wagging is orchestrated by the protein environment.<ref name="Fang">PMID: 19907490</ref> |
| - | <scene name='Green_Fluorescent_Protein/Arg96/1' >base</scene> by withdrawing electrons through hydrogen bonding with the carbonyl oxygen of Ser<sup>65</sup> to activate the carbonyl carbon for nucleophilic attack by the amide nitrogen of Gly<sup>67</sup>. This mechanism was further supported by ''ab initio'' calculations, as well as database searches of similar compounds and protein sequences. Through acid/base chemistry, the chromophore is stabilized by resonance.<ref name="Phillips" /> Femtosecond Raman spectroscopy has been used to map the alteration of the structure close the chromophore during excited-state protein transfer and shown that chromophore wagging is orchestrated by the protein environment.<ref name="Fang">PMID: 19907490</ref> | + | |
===Mutant Studies=== | ===Mutant Studies=== | ||
Revision as of 16:53, 24 August 2018
| |||||||||||
Contents |
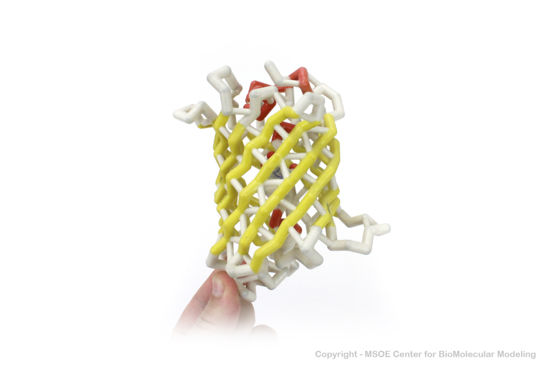
3D Printed Physical Model of Green Fluorescent Protein (GFP)
Shown below are 3D printed physical models of the Green Fluorescent Protein (GFP). The first alpha carbon backbone model is colored by three-strand repeats, including red, blue, purple, and yellow. The second alpha carbon backbone model is colored by secondary structure, with alpha helices red and beta sheets yellow. Both models show the fluorophore molecule at the center of the GFP structure.
The MSOE Center for BioMolecular Modeling
The MSOE Center for BioMolecular Modeling uses 3D printing technology to create physical models of protein and molecular structures, making the invisible molecular world more tangible and comprehensible. To view more protein structure models, visit our Model Gallery.
3D structures of Green Fluorescent Protein (Updated on 24-August-2018)
Reference for this Structure
Ormo M, Cubitt AB, Kallio K, Gross LA, Tsien RY, Remington SJ. 1996. Crystal structure of the Aequorea victoria green fluorescent protein. Science. 273(5280):1392-1395. DOI 10.1126/science.273.5280.1392.
References
- ↑ 1.0 1.1 [1], Protein Database (PDBsum): 1ema. European Bioinformatics (EBI); 2009.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 [2], Yang F, Moss LG, Phillips GN Jr. 1996. The molecular structure of green fluorescent protein. Biotechnology. 14: 1246-1251. DOI 10.1038/nbt1096-1246.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Tsien, Roger Y. 1998. The Green Fluorescent Protein. Annual Review in Biochemistry. 67:509-544.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 [3], Haldar S, Chattopadhyay A. 2009. The green journey. J Fluoresc. 19:1-2. DOI 10.1007/s10895-008-0455-6; biographical background on Douglas Prasher, Martin Chalfie and Roger Tsien.
- ↑ 5.0 5.1 5.2 5.3 [4], Shimomura O. The discovery of green fluorescent protein. Nobel Prize Lecture; 2009;; biographical background at Wikipedia.
- ↑ [5],Cowles D, Cowles J. Aequorea victoria. 2007. Walla Wall University.
- ↑ Primary structure at www.ebi.aci.uk.
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 8.11 8.12 8.13 Ormo M, Cubitt AB, Kallio K, Gross LA, Tsien RY, Remington SJ. 1996. Crystal structure of the Aequorea victoria green fluorescent protein. Science. 273(5280):1392-1395. DOI 10.1126/science.273.5280.1392.
- ↑ 9.00 9.01 9.02 9.03 9.04 9.05 9.06 9.07 9.08 9.09 9.10 9.11 9.12 9.13 Phillips GN Jr. Structure and dynamics of green fluorescent protein. Curr Opin Struct Biol. 1997 Dec;7(6):821-7. PMID:9434902
- ↑ Andrews BT, Gosavi S, Finke JM, Onuchic JN, Jennings PA. The dual-basin landscape in GFP folding. Proc Natl Acad Sci U S A. 2008 Aug 26;105(34):12283-8. Epub 2008 Aug 19. PMID:18713871
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 [6],Cubitt AB, Heim R, Adams SR, Boyd AE, Gross LA, Tsien R. 1995. Understanding, improving, and using green fluorescent protein. Trends in Biochemical Sciences. 20(11): 448-455. DOI 0.1016/S0968-0004(00)89099-4.
- ↑ Bublitz G, King BA, Boxer SG. 1998. Electronic structure of the chromophore in green fluorescent protein (GFP). Journal of the American Chemical Society. 120(36): 9370-9371. DOI 10.1021/ja98160e.
- ↑ van Thor JJ, Sage, JT. 2006. Charge transfer in green fluorescent protein. Photochemical & Photobiological Sciences. 5:597-602. DOI 10.1039/b516525c.
- ↑ 14.0 14.1 Lammich L, Petersen MA, Nielsen MB, Andersen LH. The gas-phase absorption spectrum of a neutral GFP model chromophore. Biophys J. 2007 Jan 1;92(1):201-7. Epub 2006 Oct 13. PMID:17040991 doi:10.1529/biophysj.106.093674
- ↑ Information about edge-face (CH/π) interactions.
- ↑ Fang C, Frontiera RR, Tran R, Mathies RA. Mapping GFP structure evolution during proton transfer with femtosecond Raman spectroscopy. Nature. 2009 Nov 12;462(7270):200-4. PMID:19907490 doi:10.1038/nature08527
Additional Resources
- For additional information, see: Colored & Bioluminescent Proteins
- First Glance
- PDBsum: 1ema
- RCSB PDB 1ema
- OCA
- UniProt: P42212
- Scop: P42212
- CATH: 1emaA00
- Pfam: PF01353
- InterPro: IPR000786
- GFP featured at the Molecule of the Month series of tutorials by David Goodsell.
- Inside green fluorescent protein - editor's summary that accompanied structural detail of GFP chromophore on the cover of Nature.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Wayne Decatur, Karsten Theis, Eran Hodis, Laura Carbone, Karl Oberholser, Mark Hoelzer, Joel L. Sussman, Alexander Berchansky, Jaime Prilusky, Joseph M. Steinberger, David Canner